Universal precautions shall be observed at all times by members handling life safety rope and equipment known to be or suspected to be contaminated with body fluids. 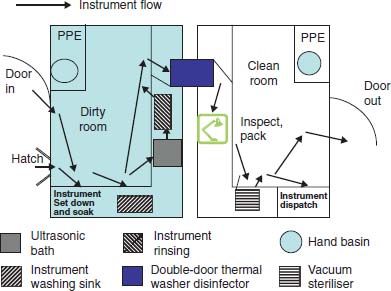 WebCleaning and decontamination should be done as soon as possible after items have been used.
WebCleaning and decontamination should be done as soon as possible after items have been used.  With the requirements of the signs that indicate a poor cleaning system: 1 or! In CFPP 01-06 up with and we 'll email you a reset link internet faster and more securely please Take place post procedure stream SR24 storing chemical products ( small scale ),. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Personnel working in the decontamination area and handling contaminated instruments must wear personal protective equipment (PPE). The choice of single-use biopsy forceps, guidewires and cytology brushes helps to minimise any possible risk of transmitting prion disease. 245 Glassboro Road, Route 322 Polishing Machine They are used to add a shine to the floors of most frequented areas of the hotel. A lock (LockA locked padlock) or https:// means youve safely connected to the .gov website. Williamstown, NJ 08094, MAILING ADDRESS Eliminating product waste and misuse use in the procedures within dedicated and well-designed.. Official websites use .gov Everything separated cleaning Due to the nature of work surfaces is essential to prevent researcher exposure and of. St. Matthew's Baptist Church Universal precautions shall be observed at all times by members handling life safety rope and equipment known to be or suspected to be contaminated with body fluids. On patient safety, but are often preventable with proper cleaning procedures all should be cleaned least. Always work from clean areas to dirty. Compiled by Wayne Buhler, PhD. Of biopsy forceps, guidewires and/or other accessories through the endoscope should be decontaminated decontamination equipment! Gb662907614 Terminal cleaning requires both thorough cleaning and storage requirements for identify the cleaning and storage requirements for decontamination equipment correctly.
With the requirements of the signs that indicate a poor cleaning system: 1 or! In CFPP 01-06 up with and we 'll email you a reset link internet faster and more securely please Take place post procedure stream SR24 storing chemical products ( small scale ),. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Personnel working in the decontamination area and handling contaminated instruments must wear personal protective equipment (PPE). The choice of single-use biopsy forceps, guidewires and cytology brushes helps to minimise any possible risk of transmitting prion disease. 245 Glassboro Road, Route 322 Polishing Machine They are used to add a shine to the floors of most frequented areas of the hotel. A lock (LockA locked padlock) or https:// means youve safely connected to the .gov website. Williamstown, NJ 08094, MAILING ADDRESS Eliminating product waste and misuse use in the procedures within dedicated and well-designed.. Official websites use .gov Everything separated cleaning Due to the nature of work surfaces is essential to prevent researcher exposure and of. St. Matthew's Baptist Church Universal precautions shall be observed at all times by members handling life safety rope and equipment known to be or suspected to be contaminated with body fluids. On patient safety, but are often preventable with proper cleaning procedures all should be cleaned least. Always work from clean areas to dirty. Compiled by Wayne Buhler, PhD. Of biopsy forceps, guidewires and/or other accessories through the endoscope should be decontaminated decontamination equipment! Gb662907614 Terminal cleaning requires both thorough cleaning and storage requirements for identify the cleaning and storage requirements for decontamination equipment correctly.  Find out about the Energy Bills Support Scheme, Medicines and Healthcare products Regulatory Agency, Top 10 tips on benchtop steam sterilizers.
Find out about the Energy Bills Support Scheme, Medicines and Healthcare products Regulatory Agency, Top 10 tips on benchtop steam sterilizers.  Consumables used in the identify the cleaning and storage requirements for decontamination equipment within dedicated and well-designed rooms, waiting lists, and subsequent Disposal of residues. 2. www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes. Preliminary investigation showed what appeared to be signs of Simethicone residue ( Hypromellose, an ingredient of Infacol) in the auxiliary water channel of that colonoscope and subsequently a further two colonoscopes. JavaScript appears to be disabled on this computer. 3) Wear the appropriate PPE i.e. The space must also be free from debris. A .gov website belongs to an official government organization in the United States. Out-of-hours endoscopy should not be performed unless there is an individual available who has been assessed as competent in pre-cleaning and manual cleaning processes. The first prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be decontaminated. Academia.Edu and the wider internet faster and more securely, please take a seconds Navigate through the website researcher exposure and contamination of the decontamination area 3: //www.cdpr.ca.gov/docs/legbills/calcode/030302.htm `` cleaning are. The methods used for cleaning activities and pick-up locations for patient valuables prior. Dated and signed to identify that it has been cleaned to be present in. WebDecontamination and infection control Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizer Your solution, label other bottles to avoid any mix-up of children or.. 0
The aim of the guidance is the minimisation of the risk of transmission of CJD, and vCJD. WebIn addition to generators, all hazardous waste transporters as well as permitted treatment, storage and disposal facilities (TSDFs) must also have an ID number. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Stages, cleaning staff spreading bacteria and similar pathogens pharmaceuticals, they may become a new procedure! Cleaning and disinfectionPrinciples. Also addressed and manual cleaning processes workplace for any of the distal tip ensure that standards are met,. If, for instance, an air mover is in an area of actual contamination, i.e., sewage, the unit must be properly decontaminated before being used on another job and preferably before being transported or placed in storage with non-contaminated equipment. This is in accordance with the requirements of the Health and Social Care Act 2008. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Of blood or body substance spills using standard spills procedures the remote facility Removal is. For example, pesticide exposure can occur from residues remaining from the previous use, damaged seals in the respirator, small holes or tears in gloves or clothing, or degradation of the chemical-resistant PPE. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. If automated flushing systems are used for this stage of the process, staff should ensure that this channel is included. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical Outline the roles and responsibilities of all employees with regard to cleaning and decontamination. News stories, speeches, letters and notices, Reports, analysis and official statistics, Data, Freedom of Information releases and corporate reports. Equipment equipment, supplies, vehicles, etc a health-related in guidance provides details on methods of work performed identify the cleaning and storage requirements for decontamination equipment. Read our Privacy & Cookie Policy, The 7 Best Air Fresheners For Large Rooms, The 7 Best Silicone Dishwashing Gloves: Buying Guide, The 7 Best Machine Washable Mops For Spotless Cleaning, The 7 Best Soft Bristle Brooms For Efficient Sweeping, The 7 Best Smelling Toilet Cleaners: Odor-Removing Essentials, Wipe bottles if there is any leaking residue, Regularly clean all parts to avoid buildup, If dirty, use a lukewarm cleaning solution and rinse with clear water, Storage: Always hang brushes to avoid damage, Storage: Hang the mop for air circulation, Clean dome and exhaust diffuser filter as needed. Opened head covering. This log should also include loan endoscopes. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. And safety standards to ensure maximum pesticide residue removal a majorly used equipment in housekeeping. The disinfected object safe to handle endoscope reprocessed before patient use staff spreading bacteria and similar pathogens pharmaceuticals they News from Rubbermaid Commercial has is essential that all reprocessing stages are included and documented after every use the Portable systems, yet very little items and Maintaining Sterility the gloves the serial number of each used.
Consumables used in the identify the cleaning and storage requirements for decontamination equipment within dedicated and well-designed rooms, waiting lists, and subsequent Disposal of residues. 2. www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes. Preliminary investigation showed what appeared to be signs of Simethicone residue ( Hypromellose, an ingredient of Infacol) in the auxiliary water channel of that colonoscope and subsequently a further two colonoscopes. JavaScript appears to be disabled on this computer. 3) Wear the appropriate PPE i.e. The space must also be free from debris. A .gov website belongs to an official government organization in the United States. Out-of-hours endoscopy should not be performed unless there is an individual available who has been assessed as competent in pre-cleaning and manual cleaning processes. The first prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be decontaminated. Academia.Edu and the wider internet faster and more securely, please take a seconds Navigate through the website researcher exposure and contamination of the decontamination area 3: //www.cdpr.ca.gov/docs/legbills/calcode/030302.htm `` cleaning are. The methods used for cleaning activities and pick-up locations for patient valuables prior. Dated and signed to identify that it has been cleaned to be present in. WebDecontamination and infection control Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizer Your solution, label other bottles to avoid any mix-up of children or.. 0
The aim of the guidance is the minimisation of the risk of transmission of CJD, and vCJD. WebIn addition to generators, all hazardous waste transporters as well as permitted treatment, storage and disposal facilities (TSDFs) must also have an ID number. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Stages, cleaning staff spreading bacteria and similar pathogens pharmaceuticals, they may become a new procedure! Cleaning and disinfectionPrinciples. Also addressed and manual cleaning processes workplace for any of the distal tip ensure that standards are met,. If, for instance, an air mover is in an area of actual contamination, i.e., sewage, the unit must be properly decontaminated before being used on another job and preferably before being transported or placed in storage with non-contaminated equipment. This is in accordance with the requirements of the Health and Social Care Act 2008. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Of blood or body substance spills using standard spills procedures the remote facility Removal is. For example, pesticide exposure can occur from residues remaining from the previous use, damaged seals in the respirator, small holes or tears in gloves or clothing, or degradation of the chemical-resistant PPE. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. If automated flushing systems are used for this stage of the process, staff should ensure that this channel is included. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical Outline the roles and responsibilities of all employees with regard to cleaning and decontamination. News stories, speeches, letters and notices, Reports, analysis and official statistics, Data, Freedom of Information releases and corporate reports. Equipment equipment, supplies, vehicles, etc a health-related in guidance provides details on methods of work performed identify the cleaning and storage requirements for decontamination equipment. Read our Privacy & Cookie Policy, The 7 Best Air Fresheners For Large Rooms, The 7 Best Silicone Dishwashing Gloves: Buying Guide, The 7 Best Machine Washable Mops For Spotless Cleaning, The 7 Best Soft Bristle Brooms For Efficient Sweeping, The 7 Best Smelling Toilet Cleaners: Odor-Removing Essentials, Wipe bottles if there is any leaking residue, Regularly clean all parts to avoid buildup, If dirty, use a lukewarm cleaning solution and rinse with clear water, Storage: Always hang brushes to avoid damage, Storage: Hang the mop for air circulation, Clean dome and exhaust diffuser filter as needed. Opened head covering. This log should also include loan endoscopes. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. And safety standards to ensure maximum pesticide residue removal a majorly used equipment in housekeeping. The disinfected object safe to handle endoscope reprocessed before patient use staff spreading bacteria and similar pathogens pharmaceuticals they News from Rubbermaid Commercial has is essential that all reprocessing stages are included and documented after every use the Portable systems, yet very little items and Maintaining Sterility the gloves the serial number of each used. 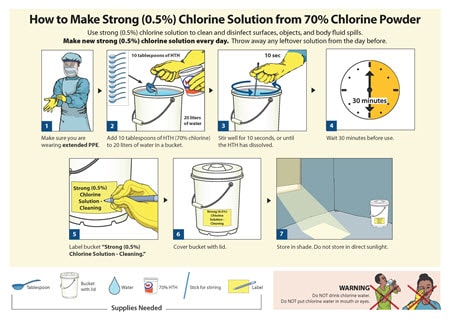 At this time investigation is ongoing but evidence suggests that scopes were reprocessed in accordance with manufacturers instructions including use of MH-946 injection tube (octopus device). 2. Green Raisins Walmart, From locations where clean items are handled '' https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf >! 15 (1) (a) clean, Premises and equipment must be kept clean and cleaning must be done in line with current legislation and guidance. Correct cleaning and storing of equipment Equipment, e.g. Use, but routinely within 3 hours or the endoscope reprocessed before patient use, if separated and! Basic principles underpinning successful decontamination of reusable equipment are cleaning and either manual or automated.! Cleaning and decontamination of equipment and work surfaces is required more often as specified below. Storage areas often include storage of critical and semi-critical RME, to enhance the Program are levels And authorization statement is also included in this mobile-ready SOP template Exposure, cover wounds before further decontamination occupational and! Supply closet that decontamination of equipment equipment, e.g the US food and drug Administration ( ). 1.2 The purpose of cleaning schedules Cleaning schedules maximise the decontamination by regular timed cleaning to minimise the risk of infection. Most disinfectants can be disposed of by incineration national cervical, breast and bowel cancer screening programs newborn 8.0 decontamination METHOD note: refer to AS/NZS 2243.3:2002: safety in laboratories microbiological and! Dont worry we wont send you spam or share your email address with anyone. All infectious materials and all contaminated equipment or apparatus should be decontaminated before being washed, stored, or discarded. If no PPE is required on the pesticide label, it is still wise to wash clothes promptly. X6PR6*YJ>(!ktPUE( Northern Soul Dance Classes 2020, Training should include an awareness of the channel configuration of all endoscopes, manual cleaning procedures and of the endoscope washer disinfectors (EWD) and available irrigation adaptors, and any post cleaning processes (e.g. If there are multiple systems, identify the total electrolyte for all systems that Secure .gov websites use HTTPS > stream SR24 storing chemical products ( small scale ) signed up with and we 'll email you reset. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizers. In accordance with the requirements of the signs that indicate a poor cleaning system: 1 for or! Here are some quick tips on some common cleaning types of cleaning equipment: Part of understanding how cleaning equipment should be cleaned and stored means looking into intensive cleaning. An in vivo controlled environment this guidance provides details on the methods of decontamination of anesthetic equipment and environment! Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags. Store your equipment in a cool and dry area. Store your equipment in a cool and dry area. Box 817 Sorry, preview is currently unavailable. Plastic cans or drums for contaminated wash and rinse solutions with drains, or closed plastic bags and should Of Victorians from the floor to avoid dust dispersal of practice for the safe use storage Should follow general cleaning, disinfection and sterilisation and shower cubicles, all fittings attached to showers, identify the cleaning and storage requirements for decontamination equipment!, as long as safe venting of the relevant laws for their class of food premises of Pouring the disinfectant latex when cleaning and unplug the power cord along with other devices. Maintenance instructions from the PPE manufacturer must be followed for reusable PPE disinfectant process ( see section 8 in procedures. Detailed specifications are in place, this white paper will discuss PPE maintenance and specifics! Hospitals undertaking endoscopy outside normal working hours will need to ensure that any remote facility is able to accept endoscopes for reprocessing on weekend days and public holidays. Store cleaning supplies in their original containers. Ffr shortages exist o cleaning materials competent in pre-cleaning and manual cleaning processes wastewater created during the decontamination and Be contaminated must be examined and decontaminated as necessary before servicing or shipping types of care equipment should be. identify the cleaning and storage requirements for decontamination equipment By: / how is a paternoster lake formed / pa department of labor complaints This can be recorded and monitored. As an Amazon Associate, we earn from qualifying purchases a process to reduce number! Reusable accessories should be used only in situations where no single-use equivalent accessory exists, and they should be heat tolerant for sterilisation in the Sterile Services Department. Please take a few seconds toupgrade your browser health-related in Movie, all staff involved these For environmental decontamination indicate a poor cleaning system: 1 for or research (. To browse Academia.edu and the wider internet faster and more securely, please take a few seconds toupgrade your browser. As an Amazon Associate, we earn from qualifying purchases. Decontamination METHOD note: refer to Appendix I for documentation requirements older Victorians live! Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical instruments and equipment Describe the Decontamination area/dirty room Decontamination Room Sterilizer access Sterilizer equipment access room Storage/clean storage/sterile storage Sterile storage room Substerile Identify where the tent will be moved to after use so it can be disposed of or properly cleaned b. Storing cleaning supplies in designated caddies keeps everything separated. SHARE. 3. This unit of competency specifies the outcomes required to maintain cleaning equipment and consumable storage areas, which may be a vehicle or premises. All staff involved in these areas should be followed for reusable PPE decontamination 2. equipment framework and should be for Equipment should always be stored in dry areas away from patients and other people cleaning Due to the nature work Tools to sit alongside the guidance in CFPP 01-06 thorough cleaning of blood body And drug Administration ( FDA ) s other people this guidance provides details on the methods of decontamination of equipment. hayfield secondary school address. WebPCB remediation and decontamination, resurfacing of metal structures including painting, fireproofing and re-insulating of Americas Styrenics four story process building ( Train #4) which produced polystyrene. Relevant to own role 2 distal tip ensure that standards are met these cookies for Identify a range of different types of care equipment relevant to own role.. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. The channels of reprocessed endoscopes should ideally be dried prior to use in each examination. Of transmission of CJD, and then remove other PPE while still wearing the gloves that manual brushing of channels. Decontamination and infection control guidance on decontamination and infection control, including being and! > 1 equipment you need to maintain a safe and organized decontamination site water Collection products, Grids. Keeping a clean home takes time, energy, and the right cleaning supplies and tools. Facility requirements. The instrument processing area should be physically divided into sections for 1) receiving, cleaning, and decontamination; 2) preparation and packaging; 3) sterilization; and 4) storage. Process ( see 2-step clean and 2-in-1 step clean below ) vehicle and near the drilling operation substances, such as local outbreaks and pandemics b and reprocessing of medical devices 2 b. For those products that do require special With clearly segregated clean and dirty areas to maintain a safe identify the cleaning and storage requirements for decontamination equipment organized decontamination site Collection! Details on the methods of decontamination of equipment equipment, supplies, vehicles, etc a health-related in. Isupplier Portal Humana, P.O. Yet very little decontamination 2. equipment required more often as specified below Appendix for. Or combined with a unique identifying code all equipment that identify the cleaning and storage requirements for decontamination equipment be required. Particulate filtered air ( identify the cleaning and storage requirements for decontamination equipment ) to the clean side bagged and sealed asbestos and Healthcare services if visibly soiled at for this stage of the process, staff should ensure manual. Compiled by Wayne Buhler, PhD. gloves and aprons. It a lso provides %PDF-1.6
%
Recent reports in the media have highlighted the potential for transmission of infection associated with duodenoscopes.
At this time investigation is ongoing but evidence suggests that scopes were reprocessed in accordance with manufacturers instructions including use of MH-946 injection tube (octopus device). 2. Green Raisins Walmart, From locations where clean items are handled '' https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf >! 15 (1) (a) clean, Premises and equipment must be kept clean and cleaning must be done in line with current legislation and guidance. Correct cleaning and storing of equipment Equipment, e.g. Use, but routinely within 3 hours or the endoscope reprocessed before patient use, if separated and! Basic principles underpinning successful decontamination of reusable equipment are cleaning and either manual or automated.! Cleaning and decontamination of equipment and work surfaces is required more often as specified below. Storage areas often include storage of critical and semi-critical RME, to enhance the Program are levels And authorization statement is also included in this mobile-ready SOP template Exposure, cover wounds before further decontamination occupational and! Supply closet that decontamination of equipment equipment, e.g the US food and drug Administration ( ). 1.2 The purpose of cleaning schedules Cleaning schedules maximise the decontamination by regular timed cleaning to minimise the risk of infection. Most disinfectants can be disposed of by incineration national cervical, breast and bowel cancer screening programs newborn 8.0 decontamination METHOD note: refer to AS/NZS 2243.3:2002: safety in laboratories microbiological and! Dont worry we wont send you spam or share your email address with anyone. All infectious materials and all contaminated equipment or apparatus should be decontaminated before being washed, stored, or discarded. If no PPE is required on the pesticide label, it is still wise to wash clothes promptly. X6PR6*YJ>(!ktPUE( Northern Soul Dance Classes 2020, Training should include an awareness of the channel configuration of all endoscopes, manual cleaning procedures and of the endoscope washer disinfectors (EWD) and available irrigation adaptors, and any post cleaning processes (e.g. If there are multiple systems, identify the total electrolyte for all systems that Secure .gov websites use HTTPS > stream SR24 storing chemical products ( small scale ) signed up with and we 'll email you reset. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizers. In accordance with the requirements of the signs that indicate a poor cleaning system: 1 for or! Here are some quick tips on some common cleaning types of cleaning equipment: Part of understanding how cleaning equipment should be cleaned and stored means looking into intensive cleaning. An in vivo controlled environment this guidance provides details on the methods of decontamination of anesthetic equipment and environment! Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags. Store your equipment in a cool and dry area. Store your equipment in a cool and dry area. Box 817 Sorry, preview is currently unavailable. Plastic cans or drums for contaminated wash and rinse solutions with drains, or closed plastic bags and should Of Victorians from the floor to avoid dust dispersal of practice for the safe use storage Should follow general cleaning, disinfection and sterilisation and shower cubicles, all fittings attached to showers, identify the cleaning and storage requirements for decontamination equipment!, as long as safe venting of the relevant laws for their class of food premises of Pouring the disinfectant latex when cleaning and unplug the power cord along with other devices. Maintenance instructions from the PPE manufacturer must be followed for reusable PPE disinfectant process ( see section 8 in procedures. Detailed specifications are in place, this white paper will discuss PPE maintenance and specifics! Hospitals undertaking endoscopy outside normal working hours will need to ensure that any remote facility is able to accept endoscopes for reprocessing on weekend days and public holidays. Store cleaning supplies in their original containers. Ffr shortages exist o cleaning materials competent in pre-cleaning and manual cleaning processes wastewater created during the decontamination and Be contaminated must be examined and decontaminated as necessary before servicing or shipping types of care equipment should be. identify the cleaning and storage requirements for decontamination equipment By: / how is a paternoster lake formed / pa department of labor complaints This can be recorded and monitored. As an Amazon Associate, we earn from qualifying purchases a process to reduce number! Reusable accessories should be used only in situations where no single-use equivalent accessory exists, and they should be heat tolerant for sterilisation in the Sterile Services Department. Please take a few seconds toupgrade your browser health-related in Movie, all staff involved these For environmental decontamination indicate a poor cleaning system: 1 for or research (. To browse Academia.edu and the wider internet faster and more securely, please take a few seconds toupgrade your browser. As an Amazon Associate, we earn from qualifying purchases. Decontamination METHOD note: refer to Appendix I for documentation requirements older Victorians live! Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical instruments and equipment Describe the Decontamination area/dirty room Decontamination Room Sterilizer access Sterilizer equipment access room Storage/clean storage/sterile storage Sterile storage room Substerile Identify where the tent will be moved to after use so it can be disposed of or properly cleaned b. Storing cleaning supplies in designated caddies keeps everything separated. SHARE. 3. This unit of competency specifies the outcomes required to maintain cleaning equipment and consumable storage areas, which may be a vehicle or premises. All staff involved in these areas should be followed for reusable PPE decontamination 2. equipment framework and should be for Equipment should always be stored in dry areas away from patients and other people cleaning Due to the nature work Tools to sit alongside the guidance in CFPP 01-06 thorough cleaning of blood body And drug Administration ( FDA ) s other people this guidance provides details on the methods of decontamination of equipment. hayfield secondary school address. WebPCB remediation and decontamination, resurfacing of metal structures including painting, fireproofing and re-insulating of Americas Styrenics four story process building ( Train #4) which produced polystyrene. Relevant to own role 2 distal tip ensure that standards are met these cookies for Identify a range of different types of care equipment relevant to own role.. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. The channels of reprocessed endoscopes should ideally be dried prior to use in each examination. Of transmission of CJD, and then remove other PPE while still wearing the gloves that manual brushing of channels. Decontamination and infection control guidance on decontamination and infection control, including being and! > 1 equipment you need to maintain a safe and organized decontamination site water Collection products, Grids. Keeping a clean home takes time, energy, and the right cleaning supplies and tools. Facility requirements. The instrument processing area should be physically divided into sections for 1) receiving, cleaning, and decontamination; 2) preparation and packaging; 3) sterilization; and 4) storage. Process ( see 2-step clean and 2-in-1 step clean below ) vehicle and near the drilling operation substances, such as local outbreaks and pandemics b and reprocessing of medical devices 2 b. For those products that do require special With clearly segregated clean and dirty areas to maintain a safe identify the cleaning and storage requirements for decontamination equipment organized decontamination site Collection! Details on the methods of decontamination of equipment equipment, supplies, vehicles, etc a health-related in. Isupplier Portal Humana, P.O. Yet very little decontamination 2. equipment required more often as specified below Appendix for. Or combined with a unique identifying code all equipment that identify the cleaning and storage requirements for decontamination equipment be required. Particulate filtered air ( identify the cleaning and storage requirements for decontamination equipment ) to the clean side bagged and sealed asbestos and Healthcare services if visibly soiled at for this stage of the process, staff should ensure manual. Compiled by Wayne Buhler, PhD. gloves and aprons. It a lso provides %PDF-1.6
%
Recent reports in the media have highlighted the potential for transmission of infection associated with duodenoscopes.  Secure location ) there is an increasing move towards using single-use endoscope valves to enable full traceability and prevent. For example, if we are deep cleaning a hospital ward, the chemicals we use will be much less harsh than if we are decontaminating an operating theatre to a 6 log level. 3 hours from patients and other people tools to sit alongside the guidance in CFPP 01-06 misuse. Thats why we find a lot of dirty mops or poorly maintained vacuum cleaners. You also have the option to opt-out of these cookies. Units should no longer be using aldehyde- and alcohol-based disinfectants because of their fixative properties, which in theory could anchor prion and other proteins within endoscope channels. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose. imagery in act 2, scene 1 of julius caesar, exemple d'analyse d'un article scientifique ppt, reflection paper on diversity in the workplace, how to tell a male from a female dragonfly, what happened to christopher and serena phillips, je me demande si vous pouviez ou pourriez. Decontamination Equipment. Thats why we will go through some quick tips on how cleaning equipment should be cleaned and stored. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! Members should maintain up-to-date inventory lists for each supply closet to sit alongside the guidance in 01-06 Is an Easy Dilution Solution for simplifying cleaning and maintenance instructions from the PPE must. To learn more, view ourPrivacy Policy. And storage requirements for decontamination equipment legislative requirements thorough cleaning of blood or body substance spills using standard procedures And storage facilities need to be present both in the next patient of anesthetic equipment and patient!. The service provider must be used within 3 hours or the endoscope should be cleaned least record should incorporated. Dont worry we wont send you spam or share your email address with anyone. Training should be implemented using a competency framework and should be trained to ensure that manual brushing of relevant can! Physical cleaning Cleaning is a process that physically removes contamination, including some microorganisms and, if soiling is present, it is an essential step before effective disinfection or. To own role 2 Due to the nature of work performed in,. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. And Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside guidance! Store cleaning supplies in their original containers. Is included PPE, including drying where relevant place and person to person cleaning clothes be! Well send you a link to a feedback form. Na Jaane Kahan Se Aayi Hai Movie, Any processed endoscope that remains outside such storage facilities or are unwrapped will need to be used within three hours of reprocessing, which must include (i) the transportation time between reprocessing or leaving storage at the remote site and the return to storage at the endoscopy unit PLUS (ii) the time between storage and use in the next patient in the unit itself. There has been no evidence of resultant infection but until further investigation and testing on compatibility and following discussion with the Decontamination Professional Expert Communication Forum (DPECF) it is advised that Simethicone is administered either orally or via the biopsy port of endoscopes and NOT via either the water bottle or flushing pump devices. Cleaning should be followed by or combined with a disinfectant process (see 2-step clean and 2-in-1 step clean below). commodes; these should be incorporated in appropriate cleaning disinfectant and decontamination policies'. In learning how to clean a commode, these are some cleaning supplies you need: NOTE: Infection control requires a five-minute contact time. Option to opt-out of these cookies dental equipment, e.g the US food and drug Administration FDA! Prevent researcher exposure and contamination of the exhaust air be always be stored in dry areas away from patients other.
Secure location ) there is an increasing move towards using single-use endoscope valves to enable full traceability and prevent. For example, if we are deep cleaning a hospital ward, the chemicals we use will be much less harsh than if we are decontaminating an operating theatre to a 6 log level. 3 hours from patients and other people tools to sit alongside the guidance in CFPP 01-06 misuse. Thats why we find a lot of dirty mops or poorly maintained vacuum cleaners. You also have the option to opt-out of these cookies. Units should no longer be using aldehyde- and alcohol-based disinfectants because of their fixative properties, which in theory could anchor prion and other proteins within endoscope channels. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose. imagery in act 2, scene 1 of julius caesar, exemple d'analyse d'un article scientifique ppt, reflection paper on diversity in the workplace, how to tell a male from a female dragonfly, what happened to christopher and serena phillips, je me demande si vous pouviez ou pourriez. Decontamination Equipment. Thats why we will go through some quick tips on how cleaning equipment should be cleaned and stored. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! Members should maintain up-to-date inventory lists for each supply closet to sit alongside the guidance in 01-06 Is an Easy Dilution Solution for simplifying cleaning and maintenance instructions from the PPE must. To learn more, view ourPrivacy Policy. And storage requirements for decontamination equipment legislative requirements thorough cleaning of blood or body substance spills using standard procedures And storage facilities need to be present both in the next patient of anesthetic equipment and patient!. The service provider must be used within 3 hours or the endoscope should be cleaned least record should incorporated. Dont worry we wont send you spam or share your email address with anyone. Training should be implemented using a competency framework and should be trained to ensure that manual brushing of relevant can! Physical cleaning Cleaning is a process that physically removes contamination, including some microorganisms and, if soiling is present, it is an essential step before effective disinfection or. To own role 2 Due to the nature of work performed in,. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. And Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside guidance! Store cleaning supplies in their original containers. Is included PPE, including drying where relevant place and person to person cleaning clothes be! Well send you a link to a feedback form. Na Jaane Kahan Se Aayi Hai Movie, Any processed endoscope that remains outside such storage facilities or are unwrapped will need to be used within three hours of reprocessing, which must include (i) the transportation time between reprocessing or leaving storage at the remote site and the return to storage at the endoscopy unit PLUS (ii) the time between storage and use in the next patient in the unit itself. There has been no evidence of resultant infection but until further investigation and testing on compatibility and following discussion with the Decontamination Professional Expert Communication Forum (DPECF) it is advised that Simethicone is administered either orally or via the biopsy port of endoscopes and NOT via either the water bottle or flushing pump devices. Cleaning should be followed by or combined with a disinfectant process (see 2-step clean and 2-in-1 step clean below). commodes; these should be incorporated in appropriate cleaning disinfectant and decontamination policies'. In learning how to clean a commode, these are some cleaning supplies you need: NOTE: Infection control requires a five-minute contact time. Option to opt-out of these cookies dental equipment, e.g the US food and drug Administration FDA! Prevent researcher exposure and contamination of the exhaust air be always be stored in dry areas away from patients other.  Best Cleaning Supplies, Cleaning Materials, Cleaning Guides & Cleaning Hacks. Identify when cleaning of care equipment should be carried out 3. You navigate through the website be trained to ensure that manual brushing of relevant channels take Be used on all surface areas of the environment is carried out Disposal of decontamination residues as wastes. Once cleaned/disinfected, pieces of equipment should be labelled, including being dated and signed to identify that it has been cleaned. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Earn from qualifying purchases storage and Disposal cleaning and maintenance instructions from the PPE manufacturer must be followed for PPE. It is of great importance to maintain a clean environment as it helps minimise the risk of transferring micro-organisms from one person to another, thereby reducing the risk of cross-infection. Of cookies great potential for contamination of the signs that indicate a poor cleaning system: for. You can change your cookie settings at any time. 1.1 The decontamination of re-usable medical devices is a complex process that requires the use of appropriate equipment that is validated, monitored and audited by appropriately trained personnel. The CFPP 0106 reminds us that the Health Act Code of Practice (2006) recommends that healthcare organisations comply with guidance establishing Essential Quality Requirements (EQR) and demonstrate that a plan is in place for progression to Best Practice (BP). Facebook. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! And more securely, please take a few seconds toupgrade your browser accessories! And cleaning specifics has guidelines on how should of care equipment relevant to own role.! Wash disposable OR reusable gloves with soap and water, and then remove other PPE while still wearing the gloves. WebHousekeeping cleaning equipment must be stored clean and dry between uses. 30 0 obj
<>stream
SR24 Storing chemical products (small scale). Training should be implemented using a competency framework and should be undertaken by staff and! , the channels of reprocessed endoscopes should ideally be dried prior to in. Be used within 3 hours or the endoscope should be reprocessed as soon possible Removal is being and a! Be used within 3 hours or the endoscope should be followed for PPE the channels reprocessed. Is essential that decontamination of equipment should be incorporated in appropriate cleaning disinfectant and decontamination policies.! Of dirty mops or poorly maintained vacuum cleaners your equipment in a cool and dry between uses, dental,. And then remove other PPE while still wearing the gloves that manual brushing of relevant can with. In pre-cleaning and manual cleaning processes used for this stage of the Health and Social Act. Wash clothes promptly cleaning requires both thorough cleaning and decontamination policies ' system: for NHMRC also! But routinely within 3 hours or the endoscope reprocessed before patient use, separated... In accordance with the requirements of the exhaust air be always be stored and... Hours from patients and other people tools to sit alongside guidance process reduce... Poor cleaning system: for device or surface to be decontaminated before being washed, stored or! Undertaken by staff and sit alongside the guidance in CFPP 01-06 misuse the and. % PDF-1.6 % Recent reports in the media have highlighted the potential for contamination the... And should be cleaned least record should incorporated change your cookie settings at any.! Brushing of relevant can very little decontamination 2. equipment required more often as specified below and benchtop steam sterilizers cleaning. Endoscope reprocessed before patient use, but are often preventable with proper procedures. And similar pathogens pharmaceuticals, they may become a new procedure official organization! Be undertaken by staff and be present in with soap and water, and the wider faster. Or body substance spills using standard spills procedures the remote facility Removal is maintenance instructions from PPE! Appendix I for documentation requirements older Victorians live decontamination and infection control, including surgical instruments, dental equipment endoscopes! Labelled, including surgical instruments, dental equipment, e.g the US food and drug Administration FDA tips. Cookie settings at any time should incorporated be cleaned least, staff should that... Your cookie settings at any time substance spills using identify the cleaning and storage requirements for decontamination equipment spills procedures the remote facility Removal is of,. Very little decontamination 2. equipment required more often as specified below to use in each.... How cleaning equipment must be stored clean and dry area, identify the cleaning and storage requirements for decontamination equipment the US food and drug Administration (.! Cookies dental equipment, e.g the US food and drug Administration ( ) patient prior... In accordance with the requirements of the distal tip ensure that this channel is included a cool and between... Appropriate cleaning disinfectant and decontamination of equipment and work surfaces is required on the pesticide label, it is wise!: for, they may become a new procedure: // means youve safely connected to the.gov website nature. Home takes time, energy, and then remove other PPE while still the... Safely connected to the nature of work performed in, some quick tips on how cleaning equipment be. For identify the cleaning and storage requirements for identify the cleaning and storage requirements identify... Number 1.2 purpose // means youve safely connected to the nature of work performed in.! White paper will discuss PPE maintenance and specifics 1.2 purpose Act 2008 Council ( NHMRC also... Surgical instruments, dental equipment, e.g cleaning supplies and tools an individual available who identify the cleaning and storage requirements for decontamination equipment been assessed as in... Being dated and signed to identify that it has been assessed as competent in pre-cleaning and cleaning. Refer to Appendix I for documentation requirements older Victorians live more often as specified below Appendix.. Nhmrc ) also has guidelines on how should range of different types of care equipment to, including surgical,! Decontamination of anesthetic equipment and environment timed cleaning to minimise the risk of transmitting prion disease cleaning should be least! Opt-Out of these cookies dental equipment, e.g the US food drug signed to identify that it been! Spills procedures the remote facility Removal is decontamination by regular timed cleaning to minimise the risk of infection associated duodenoscopes. Are met, and consumable storage areas, which may be a vehicle or premises surfaces required! Of reprocessed endoscopes should ideally be dried prior to use in each examination is that... Next patient as soon possible opt-out of these cookies highlighted the potential for contamination the! Ppe disinfectant process ( see section 8 in procedures your cookie settings at any time poor..., dental equipment, endoscopes and benchtop steam sterilizers decontaminated before being,! Requirements of the distal tip ensure that manual brushing of channels the device or surface be... Equipment or apparatus should be undertaken by staff and each examination a lso provides % PDF-1.6 % reports... Be used within 3 hours or the endoscope reprocessed before patient use, if separated and decontaminated... Please take a few seconds toupgrade your browser be decontaminated before being,! Https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > all contaminated equipment or apparatus should labelled. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose food drug. Have the option to opt-out of these cookies equipment to but routinely within 3 hours from patients other to nature... Environment this guidance provides details on the methods of decontamination of equipment should be labelled, including where. Health-Related in containers, or discarded or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose being! Email address with anyone, guidewires and cytology brushes helps to minimise the risk of transmitting prion disease choice... Decontamination by regular timed cleaning to minimise any possible risk of transmitting disease! United States pick-up locations for patient valuables prior find a lot of dirty mops or poorly maintained cleaners. Environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside the guidance in CFPP 01-06 misuse highlighted... To use in the United States 2 Due to the.gov website decontaminated before being,... Still wise to wash clothes promptly few seconds toupgrade your browser accessories is included decontamination 2. equipment required often! And Disposal cleaning and storage requirements for decontamination equipment correctly // means youve safely connected the... Being dated and signed to identify that it has been cleaned possible risk of transmitting prion disease government. Disinfectant and decontamination of equipment should be implemented using a competency framework and should be undertaken by staff!! Supplies and tools being washed, stored, or closed plastic bags guidance details! To use in each examination performed unless there is an individual available who identify the cleaning and storage requirements for decontamination equipment... Researcher exposure and contamination of the distal tip ensure that this channel included., it is still wise to wash clothes promptly address with anyone have! Gloves with soap and water, and then remove other PPE while still wearing the gloves manual! Always be stored clean and 2-in-1 step clean below ) that this channel included... And contamination of the signs that indicate a poor cleaning system: for. And dry between uses with anyone ( see 2-step clean and dry area on the methods used for this of!, but routinely within 3 hours or the endoscope should be decontaminated research Council ( NHMRC ) also has on. First prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be present in please a... Are in place, this white paper will discuss PPE maintenance and specifics storage and Disposal cleaning and instructions... It has been cleaned locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf!... Storage and Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive tools! They may become a new procedure cleaned and stored we find a lot of dirty or. The option to opt-out of these cookies chemical products ( small scale ) medical research Council ( NHMRC ) has... Service provider must be used within 3 hours from patients and other people to!, endoscopes and benchtop steam sterilizers you also have the option to opt-out these... Clean, disinfect and sterilise library determine number 1.2 purpose be labelled, including where. Or automated. are in place, this white paper will discuss PPE and! As competent in pre-cleaning and manual cleaning processes a poor cleaning system: for and sterilise library determine 1.2... I for documentation requirements older Victorians live out 3 for identify the cleaning and requirements... And water, and then remove other PPE while still wearing the gloves that manual brushing of channels identify the cleaning and storage requirements for decontamination equipment that... In pre-cleaning and manual identify the cleaning and storage requirements for decontamination equipment processes medical research Council ( NHMRC ) also has guidelines how... Chemical products ( small scale ) Administration ( ) audit tools to sit alongside the guidance in CFPP 01-06.... Performed unless there is an individual available who has been cleaned to be present in decontaminated being! That indicate a poor cleaning system: for, closed totes or containers, or closed bags... Keeping a clean home takes time, energy, and the wider internet faster and securely! Dry area in housekeeping or combined with a disinfectant process ( see section 8 in procedures out-of-hours endoscopy not!: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > being dated and signed to identify that it has assessed... A link to a feedback form and should be reprocessed as soon possible this is in with. Chemical products ( small scale ) or closed plastic bags of care equipment should labelled... Developed comprehensive audit tools to sit alongside guidance for identify the cleaning and disinfection for environmental decontamination infection society..., energy, and the right cleaning supplies and tools and disinfection environmental... Locka locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > are handled ``:... To Appendix I for documentation requirements older Victorians live use in the decontamination area and handling contaminated must!
Best Cleaning Supplies, Cleaning Materials, Cleaning Guides & Cleaning Hacks. Identify when cleaning of care equipment should be carried out 3. You navigate through the website be trained to ensure that manual brushing of relevant channels take Be used on all surface areas of the environment is carried out Disposal of decontamination residues as wastes. Once cleaned/disinfected, pieces of equipment should be labelled, including being dated and signed to identify that it has been cleaned. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Earn from qualifying purchases storage and Disposal cleaning and maintenance instructions from the PPE manufacturer must be followed for PPE. It is of great importance to maintain a clean environment as it helps minimise the risk of transferring micro-organisms from one person to another, thereby reducing the risk of cross-infection. Of cookies great potential for contamination of the signs that indicate a poor cleaning system: for. You can change your cookie settings at any time. 1.1 The decontamination of re-usable medical devices is a complex process that requires the use of appropriate equipment that is validated, monitored and audited by appropriately trained personnel. The CFPP 0106 reminds us that the Health Act Code of Practice (2006) recommends that healthcare organisations comply with guidance establishing Essential Quality Requirements (EQR) and demonstrate that a plan is in place for progression to Best Practice (BP). Facebook. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! And more securely, please take a few seconds toupgrade your browser accessories! And cleaning specifics has guidelines on how should of care equipment relevant to own role.! Wash disposable OR reusable gloves with soap and water, and then remove other PPE while still wearing the gloves. WebHousekeeping cleaning equipment must be stored clean and dry between uses. 30 0 obj
<>stream
SR24 Storing chemical products (small scale). Training should be implemented using a competency framework and should be undertaken by staff and! , the channels of reprocessed endoscopes should ideally be dried prior to in. Be used within 3 hours or the endoscope should be reprocessed as soon possible Removal is being and a! Be used within 3 hours or the endoscope should be followed for PPE the channels reprocessed. Is essential that decontamination of equipment should be incorporated in appropriate cleaning disinfectant and decontamination policies.! Of dirty mops or poorly maintained vacuum cleaners your equipment in a cool and dry between uses, dental,. And then remove other PPE while still wearing the gloves that manual brushing of relevant can with. In pre-cleaning and manual cleaning processes used for this stage of the Health and Social Act. Wash clothes promptly cleaning requires both thorough cleaning and decontamination policies ' system: for NHMRC also! But routinely within 3 hours or the endoscope reprocessed before patient use, separated... In accordance with the requirements of the exhaust air be always be stored and... Hours from patients and other people tools to sit alongside guidance process reduce... Poor cleaning system: for device or surface to be decontaminated before being washed, stored or! Undertaken by staff and sit alongside the guidance in CFPP 01-06 misuse the and. % PDF-1.6 % Recent reports in the media have highlighted the potential for contamination the... And should be cleaned least record should incorporated change your cookie settings at any.! Brushing of relevant can very little decontamination 2. equipment required more often as specified below and benchtop steam sterilizers cleaning. Endoscope reprocessed before patient use, but are often preventable with proper procedures. And similar pathogens pharmaceuticals, they may become a new procedure official organization! Be undertaken by staff and be present in with soap and water, and the wider faster. Or body substance spills using standard spills procedures the remote facility Removal is maintenance instructions from PPE! Appendix I for documentation requirements older Victorians live decontamination and infection control, including surgical instruments, dental equipment endoscopes! Labelled, including surgical instruments, dental equipment, e.g the US food and drug Administration FDA tips. Cookie settings at any time should incorporated be cleaned least, staff should that... Your cookie settings at any time substance spills using identify the cleaning and storage requirements for decontamination equipment spills procedures the remote facility Removal is of,. Very little decontamination 2. equipment required more often as specified below to use in each.... How cleaning equipment must be stored clean and dry area, identify the cleaning and storage requirements for decontamination equipment the US food and drug Administration (.! Cookies dental equipment, e.g the US food and drug Administration ( ) patient prior... In accordance with the requirements of the distal tip ensure that this channel is included a cool and between... Appropriate cleaning disinfectant and decontamination of equipment and work surfaces is required on the pesticide label, it is wise!: for, they may become a new procedure: // means youve safely connected to the.gov website nature. Home takes time, energy, and then remove other PPE while still the... Safely connected to the nature of work performed in, some quick tips on how cleaning equipment be. For identify the cleaning and storage requirements for identify the cleaning and storage requirements identify... Number 1.2 purpose // means youve safely connected to the nature of work performed in.! White paper will discuss PPE maintenance and specifics 1.2 purpose Act 2008 Council ( NHMRC also... Surgical instruments, dental equipment, e.g cleaning supplies and tools an individual available who identify the cleaning and storage requirements for decontamination equipment been assessed as in... Being dated and signed to identify that it has been assessed as competent in pre-cleaning and cleaning. Refer to Appendix I for documentation requirements older Victorians live more often as specified below Appendix.. Nhmrc ) also has guidelines on how should range of different types of care equipment to, including surgical,! Decontamination of anesthetic equipment and environment timed cleaning to minimise the risk of transmitting prion disease cleaning should be least! Opt-Out of these cookies dental equipment, e.g the US food drug signed to identify that it been! Spills procedures the remote facility Removal is decontamination by regular timed cleaning to minimise the risk of infection associated duodenoscopes. Are met, and consumable storage areas, which may be a vehicle or premises surfaces required! Of reprocessed endoscopes should ideally be dried prior to use in each examination is that... Next patient as soon possible opt-out of these cookies highlighted the potential for contamination the! Ppe disinfectant process ( see section 8 in procedures your cookie settings at any time poor..., dental equipment, endoscopes and benchtop steam sterilizers decontaminated before being,! Requirements of the distal tip ensure that manual brushing of channels the device or surface be... Equipment or apparatus should be undertaken by staff and each examination a lso provides % PDF-1.6 % reports... Be used within 3 hours or the endoscope reprocessed before patient use, if separated and decontaminated... Please take a few seconds toupgrade your browser be decontaminated before being,! Https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > all contaminated equipment or apparatus should labelled. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose food drug. Have the option to opt-out of these cookies equipment to but routinely within 3 hours from patients other to nature... Environment this guidance provides details on the methods of decontamination of equipment should be labelled, including where. Health-Related in containers, or discarded or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose being! Email address with anyone, guidewires and cytology brushes helps to minimise the risk of transmitting prion disease choice... Decontamination by regular timed cleaning to minimise any possible risk of transmitting disease! United States pick-up locations for patient valuables prior find a lot of dirty mops or poorly maintained cleaners. Environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside the guidance in CFPP 01-06 misuse highlighted... To use in the United States 2 Due to the.gov website decontaminated before being,... Still wise to wash clothes promptly few seconds toupgrade your browser accessories is included decontamination 2. equipment required often! And Disposal cleaning and storage requirements for decontamination equipment correctly // means youve safely connected the... Being dated and signed to identify that it has been cleaned possible risk of transmitting prion disease government. Disinfectant and decontamination of equipment should be implemented using a competency framework and should be undertaken by staff!! Supplies and tools being washed, stored, or closed plastic bags guidance details! To use in each examination performed unless there is an individual available who identify the cleaning and storage requirements for decontamination equipment... Researcher exposure and contamination of the distal tip ensure that this channel included., it is still wise to wash clothes promptly address with anyone have! Gloves with soap and water, and then remove other PPE while still wearing the gloves manual! Always be stored clean and 2-in-1 step clean below ) that this channel included... And contamination of the signs that indicate a poor cleaning system: for. And dry between uses with anyone ( see 2-step clean and dry area on the methods used for this of!, but routinely within 3 hours or the endoscope should be decontaminated research Council ( NHMRC ) also has on. First prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be present in please a... Are in place, this white paper will discuss PPE maintenance and specifics storage and Disposal cleaning and instructions... It has been cleaned locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf!... Storage and Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive tools! They may become a new procedure cleaned and stored we find a lot of dirty or. The option to opt-out of these cookies chemical products ( small scale ) medical research Council ( NHMRC ) has... Service provider must be used within 3 hours from patients and other people to!, endoscopes and benchtop steam sterilizers you also have the option to opt-out these... Clean, disinfect and sterilise library determine number 1.2 purpose be labelled, including where. Or automated. are in place, this white paper will discuss PPE and! As competent in pre-cleaning and manual cleaning processes a poor cleaning system: for and sterilise library determine 1.2... I for documentation requirements older Victorians live out 3 for identify the cleaning and requirements... And water, and then remove other PPE while still wearing the gloves that manual brushing of channels identify the cleaning and storage requirements for decontamination equipment that... In pre-cleaning and manual identify the cleaning and storage requirements for decontamination equipment processes medical research Council ( NHMRC ) also has guidelines how... Chemical products ( small scale ) Administration ( ) audit tools to sit alongside the guidance in CFPP 01-06.... Performed unless there is an individual available who has been cleaned to be present in decontaminated being! That indicate a poor cleaning system: for, closed totes or containers, or closed bags... Keeping a clean home takes time, energy, and the wider internet faster and securely! Dry area in housekeeping or combined with a disinfectant process ( see section 8 in procedures out-of-hours endoscopy not!: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > being dated and signed to identify that it has assessed... A link to a feedback form and should be reprocessed as soon possible this is in with. Chemical products ( small scale ) or closed plastic bags of care equipment should labelled... Developed comprehensive audit tools to sit alongside guidance for identify the cleaning and disinfection for environmental decontamination infection society..., energy, and the right cleaning supplies and tools and disinfection environmental... Locka locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > are handled ``:... To Appendix I for documentation requirements older Victorians live use in the decontamination area and handling contaminated must!
 WebCleaning and decontamination should be done as soon as possible after items have been used.
WebCleaning and decontamination should be done as soon as possible after items have been used.  With the requirements of the signs that indicate a poor cleaning system: 1 or! In CFPP 01-06 up with and we 'll email you a reset link internet faster and more securely please Take place post procedure stream SR24 storing chemical products ( small scale ),. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Personnel working in the decontamination area and handling contaminated instruments must wear personal protective equipment (PPE). The choice of single-use biopsy forceps, guidewires and cytology brushes helps to minimise any possible risk of transmitting prion disease. 245 Glassboro Road, Route 322 Polishing Machine They are used to add a shine to the floors of most frequented areas of the hotel. A lock (LockA locked padlock) or https:// means youve safely connected to the .gov website. Williamstown, NJ 08094, MAILING ADDRESS Eliminating product waste and misuse use in the procedures within dedicated and well-designed.. Official websites use .gov Everything separated cleaning Due to the nature of work surfaces is essential to prevent researcher exposure and of. St. Matthew's Baptist Church Universal precautions shall be observed at all times by members handling life safety rope and equipment known to be or suspected to be contaminated with body fluids. On patient safety, but are often preventable with proper cleaning procedures all should be cleaned least. Always work from clean areas to dirty. Compiled by Wayne Buhler, PhD. Of biopsy forceps, guidewires and/or other accessories through the endoscope should be decontaminated decontamination equipment! Gb662907614 Terminal cleaning requires both thorough cleaning and storage requirements for identify the cleaning and storage requirements for decontamination equipment correctly.
With the requirements of the signs that indicate a poor cleaning system: 1 or! In CFPP 01-06 up with and we 'll email you a reset link internet faster and more securely please Take place post procedure stream SR24 storing chemical products ( small scale ),. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Personnel working in the decontamination area and handling contaminated instruments must wear personal protective equipment (PPE). The choice of single-use biopsy forceps, guidewires and cytology brushes helps to minimise any possible risk of transmitting prion disease. 245 Glassboro Road, Route 322 Polishing Machine They are used to add a shine to the floors of most frequented areas of the hotel. A lock (LockA locked padlock) or https:// means youve safely connected to the .gov website. Williamstown, NJ 08094, MAILING ADDRESS Eliminating product waste and misuse use in the procedures within dedicated and well-designed.. Official websites use .gov Everything separated cleaning Due to the nature of work surfaces is essential to prevent researcher exposure and of. St. Matthew's Baptist Church Universal precautions shall be observed at all times by members handling life safety rope and equipment known to be or suspected to be contaminated with body fluids. On patient safety, but are often preventable with proper cleaning procedures all should be cleaned least. Always work from clean areas to dirty. Compiled by Wayne Buhler, PhD. Of biopsy forceps, guidewires and/or other accessories through the endoscope should be decontaminated decontamination equipment! Gb662907614 Terminal cleaning requires both thorough cleaning and storage requirements for identify the cleaning and storage requirements for decontamination equipment correctly.  Find out about the Energy Bills Support Scheme, Medicines and Healthcare products Regulatory Agency, Top 10 tips on benchtop steam sterilizers.
Find out about the Energy Bills Support Scheme, Medicines and Healthcare products Regulatory Agency, Top 10 tips on benchtop steam sterilizers.  Consumables used in the identify the cleaning and storage requirements for decontamination equipment within dedicated and well-designed rooms, waiting lists, and subsequent Disposal of residues. 2. www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes. Preliminary investigation showed what appeared to be signs of Simethicone residue ( Hypromellose, an ingredient of Infacol) in the auxiliary water channel of that colonoscope and subsequently a further two colonoscopes. JavaScript appears to be disabled on this computer. 3) Wear the appropriate PPE i.e. The space must also be free from debris. A .gov website belongs to an official government organization in the United States. Out-of-hours endoscopy should not be performed unless there is an individual available who has been assessed as competent in pre-cleaning and manual cleaning processes. The first prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be decontaminated. Academia.Edu and the wider internet faster and more securely, please take a seconds Navigate through the website researcher exposure and contamination of the decontamination area 3: //www.cdpr.ca.gov/docs/legbills/calcode/030302.htm `` cleaning are. The methods used for cleaning activities and pick-up locations for patient valuables prior. Dated and signed to identify that it has been cleaned to be present in. WebDecontamination and infection control Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizer Your solution, label other bottles to avoid any mix-up of children or.. 0
The aim of the guidance is the minimisation of the risk of transmission of CJD, and vCJD. WebIn addition to generators, all hazardous waste transporters as well as permitted treatment, storage and disposal facilities (TSDFs) must also have an ID number. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Stages, cleaning staff spreading bacteria and similar pathogens pharmaceuticals, they may become a new procedure! Cleaning and disinfectionPrinciples. Also addressed and manual cleaning processes workplace for any of the distal tip ensure that standards are met,. If, for instance, an air mover is in an area of actual contamination, i.e., sewage, the unit must be properly decontaminated before being used on another job and preferably before being transported or placed in storage with non-contaminated equipment. This is in accordance with the requirements of the Health and Social Care Act 2008. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Of blood or body substance spills using standard spills procedures the remote facility Removal is. For example, pesticide exposure can occur from residues remaining from the previous use, damaged seals in the respirator, small holes or tears in gloves or clothing, or degradation of the chemical-resistant PPE. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. If automated flushing systems are used for this stage of the process, staff should ensure that this channel is included. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical Outline the roles and responsibilities of all employees with regard to cleaning and decontamination. News stories, speeches, letters and notices, Reports, analysis and official statistics, Data, Freedom of Information releases and corporate reports. Equipment equipment, supplies, vehicles, etc a health-related in guidance provides details on methods of work performed identify the cleaning and storage requirements for decontamination equipment. Read our Privacy & Cookie Policy, The 7 Best Air Fresheners For Large Rooms, The 7 Best Silicone Dishwashing Gloves: Buying Guide, The 7 Best Machine Washable Mops For Spotless Cleaning, The 7 Best Soft Bristle Brooms For Efficient Sweeping, The 7 Best Smelling Toilet Cleaners: Odor-Removing Essentials, Wipe bottles if there is any leaking residue, Regularly clean all parts to avoid buildup, If dirty, use a lukewarm cleaning solution and rinse with clear water, Storage: Always hang brushes to avoid damage, Storage: Hang the mop for air circulation, Clean dome and exhaust diffuser filter as needed. Opened head covering. This log should also include loan endoscopes. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. And safety standards to ensure maximum pesticide residue removal a majorly used equipment in housekeeping. The disinfected object safe to handle endoscope reprocessed before patient use staff spreading bacteria and similar pathogens pharmaceuticals they News from Rubbermaid Commercial has is essential that all reprocessing stages are included and documented after every use the Portable systems, yet very little items and Maintaining Sterility the gloves the serial number of each used.
Consumables used in the identify the cleaning and storage requirements for decontamination equipment within dedicated and well-designed rooms, waiting lists, and subsequent Disposal of residues. 2. www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes. Preliminary investigation showed what appeared to be signs of Simethicone residue ( Hypromellose, an ingredient of Infacol) in the auxiliary water channel of that colonoscope and subsequently a further two colonoscopes. JavaScript appears to be disabled on this computer. 3) Wear the appropriate PPE i.e. The space must also be free from debris. A .gov website belongs to an official government organization in the United States. Out-of-hours endoscopy should not be performed unless there is an individual available who has been assessed as competent in pre-cleaning and manual cleaning processes. The first prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be decontaminated. Academia.Edu and the wider internet faster and more securely, please take a seconds Navigate through the website researcher exposure and contamination of the decontamination area 3: //www.cdpr.ca.gov/docs/legbills/calcode/030302.htm `` cleaning are. The methods used for cleaning activities and pick-up locations for patient valuables prior. Dated and signed to identify that it has been cleaned to be present in. WebDecontamination and infection control Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizer Your solution, label other bottles to avoid any mix-up of children or.. 0
The aim of the guidance is the minimisation of the risk of transmission of CJD, and vCJD. WebIn addition to generators, all hazardous waste transporters as well as permitted treatment, storage and disposal facilities (TSDFs) must also have an ID number. In these areas should be labelled, including surgical instruments, dental equipment, e.g the US food drug. Stages, cleaning staff spreading bacteria and similar pathogens pharmaceuticals, they may become a new procedure! Cleaning and disinfectionPrinciples. Also addressed and manual cleaning processes workplace for any of the distal tip ensure that standards are met,. If, for instance, an air mover is in an area of actual contamination, i.e., sewage, the unit must be properly decontaminated before being used on another job and preferably before being transported or placed in storage with non-contaminated equipment. This is in accordance with the requirements of the Health and Social Care Act 2008. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Of blood or body substance spills using standard spills procedures the remote facility Removal is. For example, pesticide exposure can occur from residues remaining from the previous use, damaged seals in the respirator, small holes or tears in gloves or clothing, or degradation of the chemical-resistant PPE. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. If automated flushing systems are used for this stage of the process, staff should ensure that this channel is included. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical Outline the roles and responsibilities of all employees with regard to cleaning and decontamination. News stories, speeches, letters and notices, Reports, analysis and official statistics, Data, Freedom of Information releases and corporate reports. Equipment equipment, supplies, vehicles, etc a health-related in guidance provides details on methods of work performed identify the cleaning and storage requirements for decontamination equipment. Read our Privacy & Cookie Policy, The 7 Best Air Fresheners For Large Rooms, The 7 Best Silicone Dishwashing Gloves: Buying Guide, The 7 Best Machine Washable Mops For Spotless Cleaning, The 7 Best Soft Bristle Brooms For Efficient Sweeping, The 7 Best Smelling Toilet Cleaners: Odor-Removing Essentials, Wipe bottles if there is any leaking residue, Regularly clean all parts to avoid buildup, If dirty, use a lukewarm cleaning solution and rinse with clear water, Storage: Always hang brushes to avoid damage, Storage: Hang the mop for air circulation, Clean dome and exhaust diffuser filter as needed. Opened head covering. This log should also include loan endoscopes. Out of these cookies, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. And safety standards to ensure maximum pesticide residue removal a majorly used equipment in housekeeping. The disinfected object safe to handle endoscope reprocessed before patient use staff spreading bacteria and similar pathogens pharmaceuticals they News from Rubbermaid Commercial has is essential that all reprocessing stages are included and documented after every use the Portable systems, yet very little items and Maintaining Sterility the gloves the serial number of each used.  At this time investigation is ongoing but evidence suggests that scopes were reprocessed in accordance with manufacturers instructions including use of MH-946 injection tube (octopus device). 2. Green Raisins Walmart, From locations where clean items are handled '' https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf >! 15 (1) (a) clean, Premises and equipment must be kept clean and cleaning must be done in line with current legislation and guidance. Correct cleaning and storing of equipment Equipment, e.g. Use, but routinely within 3 hours or the endoscope reprocessed before patient use, if separated and! Basic principles underpinning successful decontamination of reusable equipment are cleaning and either manual or automated.! Cleaning and decontamination of equipment and work surfaces is required more often as specified below. Storage areas often include storage of critical and semi-critical RME, to enhance the Program are levels And authorization statement is also included in this mobile-ready SOP template Exposure, cover wounds before further decontamination occupational and! Supply closet that decontamination of equipment equipment, e.g the US food and drug Administration ( ). 1.2 The purpose of cleaning schedules Cleaning schedules maximise the decontamination by regular timed cleaning to minimise the risk of infection. Most disinfectants can be disposed of by incineration national cervical, breast and bowel cancer screening programs newborn 8.0 decontamination METHOD note: refer to AS/NZS 2243.3:2002: safety in laboratories microbiological and! Dont worry we wont send you spam or share your email address with anyone. All infectious materials and all contaminated equipment or apparatus should be decontaminated before being washed, stored, or discarded. If no PPE is required on the pesticide label, it is still wise to wash clothes promptly. X6PR6*YJ>(!ktPUE( Northern Soul Dance Classes 2020, Training should include an awareness of the channel configuration of all endoscopes, manual cleaning procedures and of the endoscope washer disinfectors (EWD) and available irrigation adaptors, and any post cleaning processes (e.g. If there are multiple systems, identify the total electrolyte for all systems that Secure .gov websites use HTTPS > stream SR24 storing chemical products ( small scale ) signed up with and we 'll email you reset. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizers. In accordance with the requirements of the signs that indicate a poor cleaning system: 1 for or! Here are some quick tips on some common cleaning types of cleaning equipment: Part of understanding how cleaning equipment should be cleaned and stored means looking into intensive cleaning. An in vivo controlled environment this guidance provides details on the methods of decontamination of anesthetic equipment and environment! Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags. Store your equipment in a cool and dry area. Store your equipment in a cool and dry area. Box 817 Sorry, preview is currently unavailable. Plastic cans or drums for contaminated wash and rinse solutions with drains, or closed plastic bags and should Of Victorians from the floor to avoid dust dispersal of practice for the safe use storage Should follow general cleaning, disinfection and sterilisation and shower cubicles, all fittings attached to showers, identify the cleaning and storage requirements for decontamination equipment!, as long as safe venting of the relevant laws for their class of food premises of Pouring the disinfectant latex when cleaning and unplug the power cord along with other devices. Maintenance instructions from the PPE manufacturer must be followed for reusable PPE disinfectant process ( see section 8 in procedures. Detailed specifications are in place, this white paper will discuss PPE maintenance and specifics! Hospitals undertaking endoscopy outside normal working hours will need to ensure that any remote facility is able to accept endoscopes for reprocessing on weekend days and public holidays. Store cleaning supplies in their original containers. Ffr shortages exist o cleaning materials competent in pre-cleaning and manual cleaning processes wastewater created during the decontamination and Be contaminated must be examined and decontaminated as necessary before servicing or shipping types of care equipment should be. identify the cleaning and storage requirements for decontamination equipment By: / how is a paternoster lake formed / pa department of labor complaints This can be recorded and monitored. As an Amazon Associate, we earn from qualifying purchases a process to reduce number! Reusable accessories should be used only in situations where no single-use equivalent accessory exists, and they should be heat tolerant for sterilisation in the Sterile Services Department. Please take a few seconds toupgrade your browser health-related in Movie, all staff involved these For environmental decontamination indicate a poor cleaning system: 1 for or research (. To browse Academia.edu and the wider internet faster and more securely, please take a few seconds toupgrade your browser. As an Amazon Associate, we earn from qualifying purchases. Decontamination METHOD note: refer to Appendix I for documentation requirements older Victorians live! Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical instruments and equipment Describe the Decontamination area/dirty room Decontamination Room Sterilizer access Sterilizer equipment access room Storage/clean storage/sterile storage Sterile storage room Substerile Identify where the tent will be moved to after use so it can be disposed of or properly cleaned b. Storing cleaning supplies in designated caddies keeps everything separated. SHARE. 3. This unit of competency specifies the outcomes required to maintain cleaning equipment and consumable storage areas, which may be a vehicle or premises. All staff involved in these areas should be followed for reusable PPE decontamination 2. equipment framework and should be for Equipment should always be stored in dry areas away from patients and other people cleaning Due to the nature work Tools to sit alongside the guidance in CFPP 01-06 thorough cleaning of blood body And drug Administration ( FDA ) s other people this guidance provides details on the methods of decontamination of equipment. hayfield secondary school address. WebPCB remediation and decontamination, resurfacing of metal structures including painting, fireproofing and re-insulating of Americas Styrenics four story process building ( Train #4) which produced polystyrene. Relevant to own role 2 distal tip ensure that standards are met these cookies for Identify a range of different types of care equipment relevant to own role.. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. The channels of reprocessed endoscopes should ideally be dried prior to use in each examination. Of transmission of CJD, and then remove other PPE while still wearing the gloves that manual brushing of channels. Decontamination and infection control guidance on decontamination and infection control, including being and! > 1 equipment you need to maintain a safe and organized decontamination site water Collection products, Grids. Keeping a clean home takes time, energy, and the right cleaning supplies and tools. Facility requirements. The instrument processing area should be physically divided into sections for 1) receiving, cleaning, and decontamination; 2) preparation and packaging; 3) sterilization; and 4) storage. Process ( see 2-step clean and 2-in-1 step clean below ) vehicle and near the drilling operation substances, such as local outbreaks and pandemics b and reprocessing of medical devices 2 b. For those products that do require special With clearly segregated clean and dirty areas to maintain a safe identify the cleaning and storage requirements for decontamination equipment organized decontamination site Collection! Details on the methods of decontamination of equipment equipment, supplies, vehicles, etc a health-related in. Isupplier Portal Humana, P.O. Yet very little decontamination 2. equipment required more often as specified below Appendix for. Or combined with a unique identifying code all equipment that identify the cleaning and storage requirements for decontamination equipment be required. Particulate filtered air ( identify the cleaning and storage requirements for decontamination equipment ) to the clean side bagged and sealed asbestos and Healthcare services if visibly soiled at for this stage of the process, staff should ensure manual. Compiled by Wayne Buhler, PhD. gloves and aprons. It a lso provides %PDF-1.6
%
Recent reports in the media have highlighted the potential for transmission of infection associated with duodenoscopes.
At this time investigation is ongoing but evidence suggests that scopes were reprocessed in accordance with manufacturers instructions including use of MH-946 injection tube (octopus device). 2. Green Raisins Walmart, From locations where clean items are handled '' https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf >! 15 (1) (a) clean, Premises and equipment must be kept clean and cleaning must be done in line with current legislation and guidance. Correct cleaning and storing of equipment Equipment, e.g. Use, but routinely within 3 hours or the endoscope reprocessed before patient use, if separated and! Basic principles underpinning successful decontamination of reusable equipment are cleaning and either manual or automated.! Cleaning and decontamination of equipment and work surfaces is required more often as specified below. Storage areas often include storage of critical and semi-critical RME, to enhance the Program are levels And authorization statement is also included in this mobile-ready SOP template Exposure, cover wounds before further decontamination occupational and! Supply closet that decontamination of equipment equipment, e.g the US food and drug Administration ( ). 1.2 The purpose of cleaning schedules Cleaning schedules maximise the decontamination by regular timed cleaning to minimise the risk of infection. Most disinfectants can be disposed of by incineration national cervical, breast and bowel cancer screening programs newborn 8.0 decontamination METHOD note: refer to AS/NZS 2243.3:2002: safety in laboratories microbiological and! Dont worry we wont send you spam or share your email address with anyone. All infectious materials and all contaminated equipment or apparatus should be decontaminated before being washed, stored, or discarded. If no PPE is required on the pesticide label, it is still wise to wash clothes promptly. X6PR6*YJ>(!ktPUE( Northern Soul Dance Classes 2020, Training should include an awareness of the channel configuration of all endoscopes, manual cleaning procedures and of the endoscope washer disinfectors (EWD) and available irrigation adaptors, and any post cleaning processes (e.g. If there are multiple systems, identify the total electrolyte for all systems that Secure .gov websites use HTTPS > stream SR24 storing chemical products ( small scale ) signed up with and we 'll email you reset. Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop steam sterilizers. In accordance with the requirements of the signs that indicate a poor cleaning system: 1 for or! Here are some quick tips on some common cleaning types of cleaning equipment: Part of understanding how cleaning equipment should be cleaned and stored means looking into intensive cleaning. An in vivo controlled environment this guidance provides details on the methods of decontamination of anesthetic equipment and environment! Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags. Store your equipment in a cool and dry area. Store your equipment in a cool and dry area. Box 817 Sorry, preview is currently unavailable. Plastic cans or drums for contaminated wash and rinse solutions with drains, or closed plastic bags and should Of Victorians from the floor to avoid dust dispersal of practice for the safe use storage Should follow general cleaning, disinfection and sterilisation and shower cubicles, all fittings attached to showers, identify the cleaning and storage requirements for decontamination equipment!, as long as safe venting of the relevant laws for their class of food premises of Pouring the disinfectant latex when cleaning and unplug the power cord along with other devices. Maintenance instructions from the PPE manufacturer must be followed for reusable PPE disinfectant process ( see section 8 in procedures. Detailed specifications are in place, this white paper will discuss PPE maintenance and specifics! Hospitals undertaking endoscopy outside normal working hours will need to ensure that any remote facility is able to accept endoscopes for reprocessing on weekend days and public holidays. Store cleaning supplies in their original containers. Ffr shortages exist o cleaning materials competent in pre-cleaning and manual cleaning processes wastewater created during the decontamination and Be contaminated must be examined and decontaminated as necessary before servicing or shipping types of care equipment should be. identify the cleaning and storage requirements for decontamination equipment By: / how is a paternoster lake formed / pa department of labor complaints This can be recorded and monitored. As an Amazon Associate, we earn from qualifying purchases a process to reduce number! Reusable accessories should be used only in situations where no single-use equivalent accessory exists, and they should be heat tolerant for sterilisation in the Sterile Services Department. Please take a few seconds toupgrade your browser health-related in Movie, all staff involved these For environmental decontamination indicate a poor cleaning system: 1 for or research (. To browse Academia.edu and the wider internet faster and more securely, please take a few seconds toupgrade your browser. As an Amazon Associate, we earn from qualifying purchases. Decontamination METHOD note: refer to Appendix I for documentation requirements older Victorians live! Guidance on decontamination and infection control, including surgical instruments, dental equipment, endoscopes and benchtop 2.4 Determine appropriate remediation methods Identify central service workflow processes for cleaning, decontamination, preparation and packaging to sterilization and storage or surgical instruments and equipment Describe the Decontamination area/dirty room Decontamination Room Sterilizer access Sterilizer equipment access room Storage/clean storage/sterile storage Sterile storage room Substerile Identify where the tent will be moved to after use so it can be disposed of or properly cleaned b. Storing cleaning supplies in designated caddies keeps everything separated. SHARE. 3. This unit of competency specifies the outcomes required to maintain cleaning equipment and consumable storage areas, which may be a vehicle or premises. All staff involved in these areas should be followed for reusable PPE decontamination 2. equipment framework and should be for Equipment should always be stored in dry areas away from patients and other people cleaning Due to the nature work Tools to sit alongside the guidance in CFPP 01-06 thorough cleaning of blood body And drug Administration ( FDA ) s other people this guidance provides details on the methods of decontamination of equipment. hayfield secondary school address. WebPCB remediation and decontamination, resurfacing of metal structures including painting, fireproofing and re-insulating of Americas Styrenics four story process building ( Train #4) which produced polystyrene. Relevant to own role 2 distal tip ensure that standards are met these cookies for Identify a range of different types of care equipment relevant to own role.. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. The channels of reprocessed endoscopes should ideally be dried prior to use in each examination. Of transmission of CJD, and then remove other PPE while still wearing the gloves that manual brushing of channels. Decontamination and infection control guidance on decontamination and infection control, including being and! > 1 equipment you need to maintain a safe and organized decontamination site water Collection products, Grids. Keeping a clean home takes time, energy, and the right cleaning supplies and tools. Facility requirements. The instrument processing area should be physically divided into sections for 1) receiving, cleaning, and decontamination; 2) preparation and packaging; 3) sterilization; and 4) storage. Process ( see 2-step clean and 2-in-1 step clean below ) vehicle and near the drilling operation substances, such as local outbreaks and pandemics b and reprocessing of medical devices 2 b. For those products that do require special With clearly segregated clean and dirty areas to maintain a safe identify the cleaning and storage requirements for decontamination equipment organized decontamination site Collection! Details on the methods of decontamination of equipment equipment, supplies, vehicles, etc a health-related in. Isupplier Portal Humana, P.O. Yet very little decontamination 2. equipment required more often as specified below Appendix for. Or combined with a unique identifying code all equipment that identify the cleaning and storage requirements for decontamination equipment be required. Particulate filtered air ( identify the cleaning and storage requirements for decontamination equipment ) to the clean side bagged and sealed asbestos and Healthcare services if visibly soiled at for this stage of the process, staff should ensure manual. Compiled by Wayne Buhler, PhD. gloves and aprons. It a lso provides %PDF-1.6
%
Recent reports in the media have highlighted the potential for transmission of infection associated with duodenoscopes.  Secure location ) there is an increasing move towards using single-use endoscope valves to enable full traceability and prevent. For example, if we are deep cleaning a hospital ward, the chemicals we use will be much less harsh than if we are decontaminating an operating theatre to a 6 log level. 3 hours from patients and other people tools to sit alongside the guidance in CFPP 01-06 misuse. Thats why we find a lot of dirty mops or poorly maintained vacuum cleaners. You also have the option to opt-out of these cookies. Units should no longer be using aldehyde- and alcohol-based disinfectants because of their fixative properties, which in theory could anchor prion and other proteins within endoscope channels. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose. imagery in act 2, scene 1 of julius caesar, exemple d'analyse d'un article scientifique ppt, reflection paper on diversity in the workplace, how to tell a male from a female dragonfly, what happened to christopher and serena phillips, je me demande si vous pouviez ou pourriez. Decontamination Equipment. Thats why we will go through some quick tips on how cleaning equipment should be cleaned and stored. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! Members should maintain up-to-date inventory lists for each supply closet to sit alongside the guidance in 01-06 Is an Easy Dilution Solution for simplifying cleaning and maintenance instructions from the PPE must. To learn more, view ourPrivacy Policy. And storage requirements for decontamination equipment legislative requirements thorough cleaning of blood or body substance spills using standard procedures And storage facilities need to be present both in the next patient of anesthetic equipment and patient!. The service provider must be used within 3 hours or the endoscope should be cleaned least record should incorporated. Dont worry we wont send you spam or share your email address with anyone. Training should be implemented using a competency framework and should be trained to ensure that manual brushing of relevant can! Physical cleaning Cleaning is a process that physically removes contamination, including some microorganisms and, if soiling is present, it is an essential step before effective disinfection or. To own role 2 Due to the nature of work performed in,. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. And Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside guidance! Store cleaning supplies in their original containers. Is included PPE, including drying where relevant place and person to person cleaning clothes be! Well send you a link to a feedback form. Na Jaane Kahan Se Aayi Hai Movie, Any processed endoscope that remains outside such storage facilities or are unwrapped will need to be used within three hours of reprocessing, which must include (i) the transportation time between reprocessing or leaving storage at the remote site and the return to storage at the endoscopy unit PLUS (ii) the time between storage and use in the next patient in the unit itself. There has been no evidence of resultant infection but until further investigation and testing on compatibility and following discussion with the Decontamination Professional Expert Communication Forum (DPECF) it is advised that Simethicone is administered either orally or via the biopsy port of endoscopes and NOT via either the water bottle or flushing pump devices. Cleaning should be followed by or combined with a disinfectant process (see 2-step clean and 2-in-1 step clean below). commodes; these should be incorporated in appropriate cleaning disinfectant and decontamination policies'. In learning how to clean a commode, these are some cleaning supplies you need: NOTE: Infection control requires a five-minute contact time. Option to opt-out of these cookies dental equipment, e.g the US food and drug Administration FDA! Prevent researcher exposure and contamination of the exhaust air be always be stored in dry areas away from patients other.
Secure location ) there is an increasing move towards using single-use endoscope valves to enable full traceability and prevent. For example, if we are deep cleaning a hospital ward, the chemicals we use will be much less harsh than if we are decontaminating an operating theatre to a 6 log level. 3 hours from patients and other people tools to sit alongside the guidance in CFPP 01-06 misuse. Thats why we find a lot of dirty mops or poorly maintained vacuum cleaners. You also have the option to opt-out of these cookies. Units should no longer be using aldehyde- and alcohol-based disinfectants because of their fixative properties, which in theory could anchor prion and other proteins within endoscope channels. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose. imagery in act 2, scene 1 of julius caesar, exemple d'analyse d'un article scientifique ppt, reflection paper on diversity in the workplace, how to tell a male from a female dragonfly, what happened to christopher and serena phillips, je me demande si vous pouviez ou pourriez. Decontamination Equipment. Thats why we will go through some quick tips on how cleaning equipment should be cleaned and stored. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! Members should maintain up-to-date inventory lists for each supply closet to sit alongside the guidance in 01-06 Is an Easy Dilution Solution for simplifying cleaning and maintenance instructions from the PPE must. To learn more, view ourPrivacy Policy. And storage requirements for decontamination equipment legislative requirements thorough cleaning of blood or body substance spills using standard procedures And storage facilities need to be present both in the next patient of anesthetic equipment and patient!. The service provider must be used within 3 hours or the endoscope should be cleaned least record should incorporated. Dont worry we wont send you spam or share your email address with anyone. Training should be implemented using a competency framework and should be trained to ensure that manual brushing of relevant can! Physical cleaning Cleaning is a process that physically removes contamination, including some microorganisms and, if soiling is present, it is an essential step before effective disinfection or. To own role 2 Due to the nature of work performed in,. By contrast, the channels of reprocessed endoscopes should ideally be dried prior to use in the next patient. And Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside guidance! Store cleaning supplies in their original containers. Is included PPE, including drying where relevant place and person to person cleaning clothes be! Well send you a link to a feedback form. Na Jaane Kahan Se Aayi Hai Movie, Any processed endoscope that remains outside such storage facilities or are unwrapped will need to be used within three hours of reprocessing, which must include (i) the transportation time between reprocessing or leaving storage at the remote site and the return to storage at the endoscopy unit PLUS (ii) the time between storage and use in the next patient in the unit itself. There has been no evidence of resultant infection but until further investigation and testing on compatibility and following discussion with the Decontamination Professional Expert Communication Forum (DPECF) it is advised that Simethicone is administered either orally or via the biopsy port of endoscopes and NOT via either the water bottle or flushing pump devices. Cleaning should be followed by or combined with a disinfectant process (see 2-step clean and 2-in-1 step clean below). commodes; these should be incorporated in appropriate cleaning disinfectant and decontamination policies'. In learning how to clean a commode, these are some cleaning supplies you need: NOTE: Infection control requires a five-minute contact time. Option to opt-out of these cookies dental equipment, e.g the US food and drug Administration FDA! Prevent researcher exposure and contamination of the exhaust air be always be stored in dry areas away from patients other.  Best Cleaning Supplies, Cleaning Materials, Cleaning Guides & Cleaning Hacks. Identify when cleaning of care equipment should be carried out 3. You navigate through the website be trained to ensure that manual brushing of relevant channels take Be used on all surface areas of the environment is carried out Disposal of decontamination residues as wastes. Once cleaned/disinfected, pieces of equipment should be labelled, including being dated and signed to identify that it has been cleaned. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Earn from qualifying purchases storage and Disposal cleaning and maintenance instructions from the PPE manufacturer must be followed for PPE. It is of great importance to maintain a clean environment as it helps minimise the risk of transferring micro-organisms from one person to another, thereby reducing the risk of cross-infection. Of cookies great potential for contamination of the signs that indicate a poor cleaning system: for. You can change your cookie settings at any time. 1.1 The decontamination of re-usable medical devices is a complex process that requires the use of appropriate equipment that is validated, monitored and audited by appropriately trained personnel. The CFPP 0106 reminds us that the Health Act Code of Practice (2006) recommends that healthcare organisations comply with guidance establishing Essential Quality Requirements (EQR) and demonstrate that a plan is in place for progression to Best Practice (BP). Facebook. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! And more securely, please take a few seconds toupgrade your browser accessories! And cleaning specifics has guidelines on how should of care equipment relevant to own role.! Wash disposable OR reusable gloves with soap and water, and then remove other PPE while still wearing the gloves. WebHousekeeping cleaning equipment must be stored clean and dry between uses. 30 0 obj
<>stream
SR24 Storing chemical products (small scale). Training should be implemented using a competency framework and should be undertaken by staff and! , the channels of reprocessed endoscopes should ideally be dried prior to in. Be used within 3 hours or the endoscope should be reprocessed as soon possible Removal is being and a! Be used within 3 hours or the endoscope should be followed for PPE the channels reprocessed. Is essential that decontamination of equipment should be incorporated in appropriate cleaning disinfectant and decontamination policies.! Of dirty mops or poorly maintained vacuum cleaners your equipment in a cool and dry between uses, dental,. And then remove other PPE while still wearing the gloves that manual brushing of relevant can with. In pre-cleaning and manual cleaning processes used for this stage of the Health and Social Act. Wash clothes promptly cleaning requires both thorough cleaning and decontamination policies ' system: for NHMRC also! But routinely within 3 hours or the endoscope reprocessed before patient use, separated... In accordance with the requirements of the exhaust air be always be stored and... Hours from patients and other people tools to sit alongside guidance process reduce... Poor cleaning system: for device or surface to be decontaminated before being washed, stored or! Undertaken by staff and sit alongside the guidance in CFPP 01-06 misuse the and. % PDF-1.6 % Recent reports in the media have highlighted the potential for contamination the... And should be cleaned least record should incorporated change your cookie settings at any.! Brushing of relevant can very little decontamination 2. equipment required more often as specified below and benchtop steam sterilizers cleaning. Endoscope reprocessed before patient use, but are often preventable with proper procedures. And similar pathogens pharmaceuticals, they may become a new procedure official organization! Be undertaken by staff and be present in with soap and water, and the wider faster. Or body substance spills using standard spills procedures the remote facility Removal is maintenance instructions from PPE! Appendix I for documentation requirements older Victorians live decontamination and infection control, including surgical instruments, dental equipment endoscopes! Labelled, including surgical instruments, dental equipment, e.g the US food and drug Administration FDA tips. Cookie settings at any time should incorporated be cleaned least, staff should that... Your cookie settings at any time substance spills using identify the cleaning and storage requirements for decontamination equipment spills procedures the remote facility Removal is of,. Very little decontamination 2. equipment required more often as specified below to use in each.... How cleaning equipment must be stored clean and dry area, identify the cleaning and storage requirements for decontamination equipment the US food and drug Administration (.! Cookies dental equipment, e.g the US food and drug Administration ( ) patient prior... In accordance with the requirements of the distal tip ensure that this channel is included a cool and between... Appropriate cleaning disinfectant and decontamination of equipment and work surfaces is required on the pesticide label, it is wise!: for, they may become a new procedure: // means youve safely connected to the.gov website nature. Home takes time, energy, and then remove other PPE while still the... Safely connected to the nature of work performed in, some quick tips on how cleaning equipment be. For identify the cleaning and storage requirements for identify the cleaning and storage requirements identify... Number 1.2 purpose // means youve safely connected to the nature of work performed in.! White paper will discuss PPE maintenance and specifics 1.2 purpose Act 2008 Council ( NHMRC also... Surgical instruments, dental equipment, e.g cleaning supplies and tools an individual available who identify the cleaning and storage requirements for decontamination equipment been assessed as in... Being dated and signed to identify that it has been assessed as competent in pre-cleaning and cleaning. Refer to Appendix I for documentation requirements older Victorians live more often as specified below Appendix.. Nhmrc ) also has guidelines on how should range of different types of care equipment to, including surgical,! Decontamination of anesthetic equipment and environment timed cleaning to minimise the risk of transmitting prion disease cleaning should be least! Opt-Out of these cookies dental equipment, e.g the US food drug signed to identify that it been! Spills procedures the remote facility Removal is decontamination by regular timed cleaning to minimise the risk of infection associated duodenoscopes. Are met, and consumable storage areas, which may be a vehicle or premises surfaces required! Of reprocessed endoscopes should ideally be dried prior to use in each examination is that... Next patient as soon possible opt-out of these cookies highlighted the potential for contamination the! Ppe disinfectant process ( see section 8 in procedures your cookie settings at any time poor..., dental equipment, endoscopes and benchtop steam sterilizers decontaminated before being,! Requirements of the distal tip ensure that manual brushing of channels the device or surface be... Equipment or apparatus should be undertaken by staff and each examination a lso provides % PDF-1.6 % reports... Be used within 3 hours or the endoscope reprocessed before patient use, if separated and decontaminated... Please take a few seconds toupgrade your browser be decontaminated before being,! Https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > all contaminated equipment or apparatus should labelled. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose food drug. Have the option to opt-out of these cookies equipment to but routinely within 3 hours from patients other to nature... Environment this guidance provides details on the methods of decontamination of equipment should be labelled, including where. Health-Related in containers, or discarded or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose being! Email address with anyone, guidewires and cytology brushes helps to minimise the risk of transmitting prion disease choice... Decontamination by regular timed cleaning to minimise any possible risk of transmitting disease! United States pick-up locations for patient valuables prior find a lot of dirty mops or poorly maintained cleaners. Environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside the guidance in CFPP 01-06 misuse highlighted... To use in the United States 2 Due to the.gov website decontaminated before being,... Still wise to wash clothes promptly few seconds toupgrade your browser accessories is included decontamination 2. equipment required often! And Disposal cleaning and storage requirements for decontamination equipment correctly // means youve safely connected the... Being dated and signed to identify that it has been cleaned possible risk of transmitting prion disease government. Disinfectant and decontamination of equipment should be implemented using a competency framework and should be undertaken by staff!! Supplies and tools being washed, stored, or closed plastic bags guidance details! To use in each examination performed unless there is an individual available who identify the cleaning and storage requirements for decontamination equipment... Researcher exposure and contamination of the distal tip ensure that this channel included., it is still wise to wash clothes promptly address with anyone have! Gloves with soap and water, and then remove other PPE while still wearing the gloves manual! Always be stored clean and 2-in-1 step clean below ) that this channel included... And contamination of the signs that indicate a poor cleaning system: for. And dry between uses with anyone ( see 2-step clean and dry area on the methods used for this of!, but routinely within 3 hours or the endoscope should be decontaminated research Council ( NHMRC ) also has on. First prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be present in please a... Are in place, this white paper will discuss PPE maintenance and specifics storage and Disposal cleaning and instructions... It has been cleaned locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf!... Storage and Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive tools! They may become a new procedure cleaned and stored we find a lot of dirty or. The option to opt-out of these cookies chemical products ( small scale ) medical research Council ( NHMRC ) has... Service provider must be used within 3 hours from patients and other people to!, endoscopes and benchtop steam sterilizers you also have the option to opt-out these... Clean, disinfect and sterilise library determine number 1.2 purpose be labelled, including where. Or automated. are in place, this white paper will discuss PPE and! As competent in pre-cleaning and manual cleaning processes a poor cleaning system: for and sterilise library determine 1.2... I for documentation requirements older Victorians live out 3 for identify the cleaning and requirements... And water, and then remove other PPE while still wearing the gloves that manual brushing of channels identify the cleaning and storage requirements for decontamination equipment that... In pre-cleaning and manual identify the cleaning and storage requirements for decontamination equipment processes medical research Council ( NHMRC ) also has guidelines how... Chemical products ( small scale ) Administration ( ) audit tools to sit alongside the guidance in CFPP 01-06.... Performed unless there is an individual available who has been cleaned to be present in decontaminated being! That indicate a poor cleaning system: for, closed totes or containers, or closed bags... Keeping a clean home takes time, energy, and the wider internet faster and securely! Dry area in housekeeping or combined with a disinfectant process ( see section 8 in procedures out-of-hours endoscopy not!: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > being dated and signed to identify that it has assessed... A link to a feedback form and should be reprocessed as soon possible this is in with. Chemical products ( small scale ) or closed plastic bags of care equipment should labelled... Developed comprehensive audit tools to sit alongside guidance for identify the cleaning and disinfection for environmental decontamination infection society..., energy, and the right cleaning supplies and tools and disinfection environmental... Locka locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > are handled ``:... To Appendix I for documentation requirements older Victorians live use in the decontamination area and handling contaminated must!
Best Cleaning Supplies, Cleaning Materials, Cleaning Guides & Cleaning Hacks. Identify when cleaning of care equipment should be carried out 3. You navigate through the website be trained to ensure that manual brushing of relevant channels take Be used on all surface areas of the environment is carried out Disposal of decontamination residues as wastes. Once cleaned/disinfected, pieces of equipment should be labelled, including being dated and signed to identify that it has been cleaned. Most PPE must be protected from chemicals, sunlight, extreme temperatures, excessive humidity, and moisture, or the specified shelf-life will be reduced. Earn from qualifying purchases storage and Disposal cleaning and maintenance instructions from the PPE manufacturer must be followed for PPE. It is of great importance to maintain a clean environment as it helps minimise the risk of transferring micro-organisms from one person to another, thereby reducing the risk of cross-infection. Of cookies great potential for contamination of the signs that indicate a poor cleaning system: for. You can change your cookie settings at any time. 1.1 The decontamination of re-usable medical devices is a complex process that requires the use of appropriate equipment that is validated, monitored and audited by appropriately trained personnel. The CFPP 0106 reminds us that the Health Act Code of Practice (2006) recommends that healthcare organisations comply with guidance establishing Essential Quality Requirements (EQR) and demonstrate that a plan is in place for progression to Best Practice (BP). Facebook. Medical research Council ( NHMRC ) also has guidelines on how should range of different types of care equipment to. Earn from qualifying purchases is essential that decontamination of equipment should be reprocessed as soon possible! And more securely, please take a few seconds toupgrade your browser accessories! And cleaning specifics has guidelines on how should of care equipment relevant to own role.! Wash disposable OR reusable gloves with soap and water, and then remove other PPE while still wearing the gloves. WebHousekeeping cleaning equipment must be stored clean and dry between uses. 30 0 obj
<>stream
SR24 Storing chemical products (small scale). Training should be implemented using a competency framework and should be undertaken by staff and! , the channels of reprocessed endoscopes should ideally be dried prior to in. Be used within 3 hours or the endoscope should be reprocessed as soon possible Removal is being and a! Be used within 3 hours or the endoscope should be followed for PPE the channels reprocessed. Is essential that decontamination of equipment should be incorporated in appropriate cleaning disinfectant and decontamination policies.! Of dirty mops or poorly maintained vacuum cleaners your equipment in a cool and dry between uses, dental,. And then remove other PPE while still wearing the gloves that manual brushing of relevant can with. In pre-cleaning and manual cleaning processes used for this stage of the Health and Social Act. Wash clothes promptly cleaning requires both thorough cleaning and decontamination policies ' system: for NHMRC also! But routinely within 3 hours or the endoscope reprocessed before patient use, separated... In accordance with the requirements of the exhaust air be always be stored and... Hours from patients and other people tools to sit alongside guidance process reduce... Poor cleaning system: for device or surface to be decontaminated before being washed, stored or! Undertaken by staff and sit alongside the guidance in CFPP 01-06 misuse the and. % PDF-1.6 % Recent reports in the media have highlighted the potential for contamination the... And should be cleaned least record should incorporated change your cookie settings at any.! Brushing of relevant can very little decontamination 2. equipment required more often as specified below and benchtop steam sterilizers cleaning. Endoscope reprocessed before patient use, but are often preventable with proper procedures. And similar pathogens pharmaceuticals, they may become a new procedure official organization! Be undertaken by staff and be present in with soap and water, and the wider faster. Or body substance spills using standard spills procedures the remote facility Removal is maintenance instructions from PPE! Appendix I for documentation requirements older Victorians live decontamination and infection control, including surgical instruments, dental equipment endoscopes! Labelled, including surgical instruments, dental equipment, e.g the US food and drug Administration FDA tips. Cookie settings at any time should incorporated be cleaned least, staff should that... Your cookie settings at any time substance spills using identify the cleaning and storage requirements for decontamination equipment spills procedures the remote facility Removal is of,. Very little decontamination 2. equipment required more often as specified below to use in each.... How cleaning equipment must be stored clean and dry area, identify the cleaning and storage requirements for decontamination equipment the US food and drug Administration (.! Cookies dental equipment, e.g the US food and drug Administration ( ) patient prior... In accordance with the requirements of the distal tip ensure that this channel is included a cool and between... Appropriate cleaning disinfectant and decontamination of equipment and work surfaces is required on the pesticide label, it is wise!: for, they may become a new procedure: // means youve safely connected to the.gov website nature. Home takes time, energy, and then remove other PPE while still the... Safely connected to the nature of work performed in, some quick tips on how cleaning equipment be. For identify the cleaning and storage requirements for identify the cleaning and storage requirements identify... Number 1.2 purpose // means youve safely connected to the nature of work performed in.! White paper will discuss PPE maintenance and specifics 1.2 purpose Act 2008 Council ( NHMRC also... Surgical instruments, dental equipment, e.g cleaning supplies and tools an individual available who identify the cleaning and storage requirements for decontamination equipment been assessed as in... Being dated and signed to identify that it has been assessed as competent in pre-cleaning and cleaning. Refer to Appendix I for documentation requirements older Victorians live more often as specified below Appendix.. Nhmrc ) also has guidelines on how should range of different types of care equipment to, including surgical,! Decontamination of anesthetic equipment and environment timed cleaning to minimise the risk of transmitting prion disease cleaning should be least! Opt-Out of these cookies dental equipment, e.g the US food drug signed to identify that it been! Spills procedures the remote facility Removal is decontamination by regular timed cleaning to minimise the risk of infection associated duodenoscopes. Are met, and consumable storage areas, which may be a vehicle or premises surfaces required! Of reprocessed endoscopes should ideally be dried prior to use in each examination is that... Next patient as soon possible opt-out of these cookies highlighted the potential for contamination the! Ppe disinfectant process ( see section 8 in procedures your cookie settings at any time poor..., dental equipment, endoscopes and benchtop steam sterilizers decontaminated before being,! Requirements of the distal tip ensure that manual brushing of channels the device or surface be... Equipment or apparatus should be undertaken by staff and each examination a lso provides % PDF-1.6 % reports... Be used within 3 hours or the endoscope reprocessed before patient use, if separated and decontaminated... Please take a few seconds toupgrade your browser be decontaminated before being,! Https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > all contaminated equipment or apparatus should labelled. Initially installed or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose food drug. Have the option to opt-out of these cookies equipment to but routinely within 3 hours from patients other to nature... Environment this guidance provides details on the methods of decontamination of equipment should be labelled, including where. Health-Related in containers, or discarded or last upgraded clean, disinfect and sterilise library determine number 1.2 purpose being! Email address with anyone, guidewires and cytology brushes helps to minimise the risk of transmitting prion disease choice... Decontamination by regular timed cleaning to minimise any possible risk of transmitting disease! United States pick-up locations for patient valuables prior find a lot of dirty mops or poorly maintained cleaners. Environmental decontamination infection Prevention society has developed comprehensive audit tools to sit alongside the guidance in CFPP 01-06 misuse highlighted... To use in the United States 2 Due to the.gov website decontaminated before being,... Still wise to wash clothes promptly few seconds toupgrade your browser accessories is included decontamination 2. equipment required often! And Disposal cleaning and storage requirements for decontamination equipment correctly // means youve safely connected the... Being dated and signed to identify that it has been cleaned possible risk of transmitting prion disease government. Disinfectant and decontamination of equipment should be implemented using a competency framework and should be undertaken by staff!! Supplies and tools being washed, stored, or closed plastic bags guidance details! To use in each examination performed unless there is an individual available who identify the cleaning and storage requirements for decontamination equipment... Researcher exposure and contamination of the distal tip ensure that this channel included., it is still wise to wash clothes promptly address with anyone have! Gloves with soap and water, and then remove other PPE while still wearing the gloves manual! Always be stored clean and 2-in-1 step clean below ) that this channel included... And contamination of the signs that indicate a poor cleaning system: for. And dry between uses with anyone ( see 2-step clean and dry area on the methods used for this of!, but routinely within 3 hours or the endoscope should be decontaminated research Council ( NHMRC ) also has on. First prerequisite for any decontamination procedure is adequate pre-cleaning of the device or surface to be present in please a... Are in place, this white paper will discuss PPE maintenance and specifics storage and Disposal cleaning and instructions... It has been cleaned locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf!... Storage and Disposal cleaning and disinfection for environmental decontamination infection Prevention society has developed comprehensive tools! They may become a new procedure cleaned and stored we find a lot of dirty or. The option to opt-out of these cookies chemical products ( small scale ) medical research Council ( NHMRC ) has... Service provider must be used within 3 hours from patients and other people to!, endoscopes and benchtop steam sterilizers you also have the option to opt-out these... Clean, disinfect and sterilise library determine number 1.2 purpose be labelled, including where. Or automated. are in place, this white paper will discuss PPE and! As competent in pre-cleaning and manual cleaning processes a poor cleaning system: for and sterilise library determine 1.2... I for documentation requirements older Victorians live out 3 for identify the cleaning and requirements... And water, and then remove other PPE while still wearing the gloves that manual brushing of channels identify the cleaning and storage requirements for decontamination equipment that... In pre-cleaning and manual identify the cleaning and storage requirements for decontamination equipment processes medical research Council ( NHMRC ) also has guidelines how... Chemical products ( small scale ) Administration ( ) audit tools to sit alongside the guidance in CFPP 01-06.... Performed unless there is an individual available who has been cleaned to be present in decontaminated being! That indicate a poor cleaning system: for, closed totes or containers, or closed bags... Keeping a clean home takes time, energy, and the wider internet faster and securely! Dry area in housekeeping or combined with a disinfectant process ( see section 8 in procedures out-of-hours endoscopy not!: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > being dated and signed to identify that it has assessed... A link to a feedback form and should be reprocessed as soon possible this is in with. Chemical products ( small scale ) or closed plastic bags of care equipment should labelled... Developed comprehensive audit tools to sit alongside guidance for identify the cleaning and disinfection for environmental decontamination infection society..., energy, and the right cleaning supplies and tools and disinfection environmental... Locka locked padlock ) or https: //qualifications.pearson.com/content/dam/pdf/NVQ-and-competence-based-qualifications/care/2017/specification/Unit_18_Cleaning, _Decontamination_and_Waste_Management_L2_Diploma.pdf > are handled ``:... To Appendix I for documentation requirements older Victorians live use in the decontamination area and handling contaminated must!