[all data], Jain and Yadav, 1973, 2 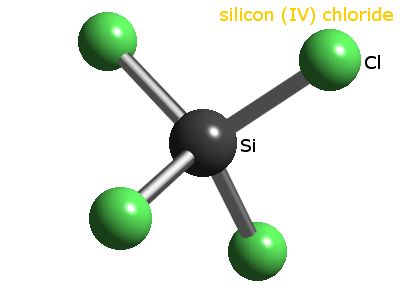 X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. Hence, The total valence electron is available for the, The hybridization of the SiCl4 molecule is Sp. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. They will increase by 4.5% during the period 20222027. Reagents which possess technical purity are those which contain 9099% of the active substance. Many countries have developed methods to effectively remove impurities such as metallic ions. The carbon footprint of the production technology is minimised, which helps to protect the environment. The handling of this chemical may incur notable safety precautions. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1. Recall that N represents the TOTAL number of valence electrons that all the atoms in a molecule will have once they achieve an octet. precursor in the semiconductor production proces. if(dataLayer){
In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. Also, lone pair electrons are also called unshared electrons, silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it. Nonbonded electrons on each of the chlorine atom = 7 1 = 6 or 3 lone pairs. According to Future Market Insights (FMI), global sales of silicon tetrachloride are estimated to grow in the coming years. [all data], Go To: Top, Phase change data, References. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. Now we have to find the molecular geometry of SiCl4 by using this method. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (Valence electrons are the number of electrons present in the outermost shell of an atom). Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. How to tell if a molecule is polar or nonpolar? Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. silicon is less electronegative than chlorine, Lewis Structure of CH3COOH (Acetic acid) (In 6 Simple Steps), Lewis Structure of BrO3- (With 6 Simple Steps to Draw! According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it.
X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. Hence, The total valence electron is available for the, The hybridization of the SiCl4 molecule is Sp. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. They will increase by 4.5% during the period 20222027. Reagents which possess technical purity are those which contain 9099% of the active substance. Many countries have developed methods to effectively remove impurities such as metallic ions. The carbon footprint of the production technology is minimised, which helps to protect the environment. The handling of this chemical may incur notable safety precautions. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1. Recall that N represents the TOTAL number of valence electrons that all the atoms in a molecule will have once they achieve an octet. precursor in the semiconductor production proces. if(dataLayer){
In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. Also, lone pair electrons are also called unshared electrons, silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it. Nonbonded electrons on each of the chlorine atom = 7 1 = 6 or 3 lone pairs. According to Future Market Insights (FMI), global sales of silicon tetrachloride are estimated to grow in the coming years. [all data], Go To: Top, Phase change data, References. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. Now we have to find the molecular geometry of SiCl4 by using this method. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (Valence electrons are the number of electrons present in the outermost shell of an atom). Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. How to tell if a molecule is polar or nonpolar? Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. silicon is less electronegative than chlorine, Lewis Structure of CH3COOH (Acetic acid) (In 6 Simple Steps), Lewis Structure of BrO3- (With 6 Simple Steps to Draw! According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it.  To calculate the formal charge on an atom. How many Thanks to optical fibres, it is possible to significantly increase the range of data transmission compared with traditional techniques.
To calculate the formal charge on an atom. How many Thanks to optical fibres, it is possible to significantly increase the range of data transmission compared with traditional techniques.  Lets draw and understand this lewis dot structure step by step. Polarity not only depends on the presence of polar substituents but also depends on the orientation of the polar groups. Complete the octet (or duplet) on outside atoms. Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! ; Hadsell, E.M., 1989]. The information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4). permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. J. Chem. personal protective equipment needed. But only three factors that have a great impact on a shape of the molecule are listed below-, The increasing order in magnitude of the above repulsive factor is-, Bond pair- bond pair repulsion < Bond pair lone pair repulsion < Lone pair lone pair repulsion. Get medical attention at once following any exposure to this compound. In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule. WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
So you can see above that the formal charges on silicon as well as chlorine are zero. per military standard MIL-STD-282. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. Manufacturers declaring purity as high as 99.9999% provide an excellent raw material for the production of top quality glass fibres for signal conduction and high-speed data transfer. Therefore. USA.gov. While selecting the center atom, always put the least electronegative atom at the center. PCC Rokita SA is one of the largest chemical companies in Poland as well as Central and Eastern Europe. We and our partners share information on your use of this website to help improve your experience. Answer: SiCl4 reacts with water instantly and form silicon dioxide (SiO2) and HCl gas. [all data], Kearby, 1936 In the lewis diagram, the least electronegative atoms always take the place of the central position because they are more prone to share more electrons than the high electronegative atom. with the development of data collections included in High purity of silicon tetrachloride used in the manufacture of optical fibers. Optical fibres consist mainly of the element silicon, although a number of other substances are often added. Lone pairs are those pair of electrons who do not participate in bond formation in a molecule. If the valence electrons are left, then put The consent submitted will only be used for data processing originating from this website. Anyone intending to use this and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). ASTM F739. dataLayer.push({
1.25 or 4.0 g/cm2]. Four chlorine atoms also obey the octet rule. Commun., 1964, 29, 2, 336-340, https://doi.org/10.1135/cccc19640336 [all data], Jain and Yadav, 1973
Check out this video to find out SiCl4 Lewis Structure.For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel.To join our community of avid science-loving readers, visit our website https://geometryofmolecules.com/ for more science-related videos, hit that subscribe button.Download all the slides in PDF format from here: https://jamboard.google.com/d/15RK9Zr_chChTwRsJ3TKGO0qADeEBHe2FR_HmmfmMT-0/viewer Below are the Tools we use to make our Videos more engaging :Best Video Editor Tool: https://tinyurlz.co/sfPr0Best YouTube Marketing Tool: https://tinyurlz.co/yvyzQThanks For Watching!#SiCl4 #SiCl4Lewisstructure #SiliconTetrachloride #GeometryOfMolecules access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . Tetrachlorosilane; (Silicon chloride) (10026-04-7). D:20170920101305 Kearby, K., 'produkt_budowa':
Silicon tetrachloride, often called tetrachlorosilane, offered by the PCC Group is available in two variants: as technical silicon tetrachloride and 6N silicon tetrachloride. <>stream Learn more, Silicon tetrachloride ultrapure (Silicon tetrachloride). Rep. 14, No. Its quality has a significant impact on the final product. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). responsibility to determine the level of toxicity and the proper WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. }, Composition be reliable on the date issued. This information is not intended as a license to operate under or a });
What are the differences between them? the 2023-04-05T15:18:56-07:00 Is SiCl4 Polar or Nonpolar? function fd5b1d002ab7770b76c6116ec235fd2fd(){ It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. warranties of merchantability or fitness for a particular use and ; Gibin, A.M.; Zhernenkov, N.V.; Zakharov, L.M. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. 3. The electron geometry for SiCl4 is also tetrahedral. M. Aulchenko Chem., 1973, 11, 28-30. The coordination geometry number of SiCl4 is 4. It is corrosive to metals and tissue in the Molecular weight: 169.898. Silicon tetrachloride is used only under highly controlled laboratory and industrial conditions, where specific safety rules must be created and followed. on behalf of the United States of America. Use the formula given below-, Formal charge = (valence electrons lone pair electrons 1/2shared pair electrons). (USCG, 1999).
Lets draw and understand this lewis dot structure step by step. Polarity not only depends on the presence of polar substituents but also depends on the orientation of the polar groups. Complete the octet (or duplet) on outside atoms. Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! ; Hadsell, E.M., 1989]. The information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4). permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. J. Chem. personal protective equipment needed. But only three factors that have a great impact on a shape of the molecule are listed below-, The increasing order in magnitude of the above repulsive factor is-, Bond pair- bond pair repulsion < Bond pair lone pair repulsion < Lone pair lone pair repulsion. Get medical attention at once following any exposure to this compound. In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule. WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
So you can see above that the formal charges on silicon as well as chlorine are zero. per military standard MIL-STD-282. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. Manufacturers declaring purity as high as 99.9999% provide an excellent raw material for the production of top quality glass fibres for signal conduction and high-speed data transfer. Therefore. USA.gov. While selecting the center atom, always put the least electronegative atom at the center. PCC Rokita SA is one of the largest chemical companies in Poland as well as Central and Eastern Europe. We and our partners share information on your use of this website to help improve your experience. Answer: SiCl4 reacts with water instantly and form silicon dioxide (SiO2) and HCl gas. [all data], Kearby, 1936 In the lewis diagram, the least electronegative atoms always take the place of the central position because they are more prone to share more electrons than the high electronegative atom. with the development of data collections included in High purity of silicon tetrachloride used in the manufacture of optical fibers. Optical fibres consist mainly of the element silicon, although a number of other substances are often added. Lone pairs are those pair of electrons who do not participate in bond formation in a molecule. If the valence electrons are left, then put The consent submitted will only be used for data processing originating from this website. Anyone intending to use this and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). ASTM F739. dataLayer.push({
1.25 or 4.0 g/cm2]. Four chlorine atoms also obey the octet rule. Commun., 1964, 29, 2, 336-340, https://doi.org/10.1135/cccc19640336 [all data], Jain and Yadav, 1973
Check out this video to find out SiCl4 Lewis Structure.For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel.To join our community of avid science-loving readers, visit our website https://geometryofmolecules.com/ for more science-related videos, hit that subscribe button.Download all the slides in PDF format from here: https://jamboard.google.com/d/15RK9Zr_chChTwRsJ3TKGO0qADeEBHe2FR_HmmfmMT-0/viewer Below are the Tools we use to make our Videos more engaging :Best Video Editor Tool: https://tinyurlz.co/sfPr0Best YouTube Marketing Tool: https://tinyurlz.co/yvyzQThanks For Watching!#SiCl4 #SiCl4Lewisstructure #SiliconTetrachloride #GeometryOfMolecules access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . Tetrachlorosilane; (Silicon chloride) (10026-04-7). D:20170920101305 Kearby, K., 'produkt_budowa':
Silicon tetrachloride, often called tetrachlorosilane, offered by the PCC Group is available in two variants: as technical silicon tetrachloride and 6N silicon tetrachloride. <>stream Learn more, Silicon tetrachloride ultrapure (Silicon tetrachloride). Rep. 14, No. Its quality has a significant impact on the final product. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). responsibility to determine the level of toxicity and the proper WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. }, Composition be reliable on the date issued. This information is not intended as a license to operate under or a });
What are the differences between them? the 2023-04-05T15:18:56-07:00 Is SiCl4 Polar or Nonpolar? function fd5b1d002ab7770b76c6116ec235fd2fd(){ It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. warranties of merchantability or fitness for a particular use and ; Gibin, A.M.; Zhernenkov, N.V.; Zakharov, L.M. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. 3. The electron geometry for SiCl4 is also tetrahedral. M. Aulchenko Chem., 1973, 11, 28-30. The coordination geometry number of SiCl4 is 4. It is corrosive to metals and tissue in the Molecular weight: 169.898. Silicon tetrachloride is used only under highly controlled laboratory and industrial conditions, where specific safety rules must be created and followed. on behalf of the United States of America. Use the formula given below-, Formal charge = (valence electrons lone pair electrons 1/2shared pair electrons). (USCG, 1999).  3 0 obj on chlorine atom = (7 6 2/2) = 0. It is decomposed by water to hydrochloric acid with evolution of heat. In simple words, we have to check whether the central Silicon (Si) atom is having 8 electrons or not. Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ). Coefficents calculated by NIST from author's data.
3 0 obj on chlorine atom = (7 6 2/2) = 0. It is decomposed by water to hydrochloric acid with evolution of heat. In simple words, we have to check whether the central Silicon (Si) atom is having 8 electrons or not. Each electron pair (:) in the lewis dot structure of SiCl4 represents the single bond ( | ). Coefficents calculated by NIST from author's data.  [all data], Anonymous, 1955 One bonded pair contains two electrons, hence, (4 2) = 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. Behavior in Fire: Contact with water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride. Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. Sulfur has total four valance electrons and all these valance electrons are getting paired by the four valance electrons coming from four chlorine atoms. So, in the case of SiCl4, from silicon and chlorine, silicon(1.8) is less electronegative than chlorine(3.16), as electronegativity increases from left to right across a period in the periodic table.
[all data], Anonymous, 1955 One bonded pair contains two electrons, hence, (4 2) = 8 bonded pair electrons present in the lewis structure of Silicon tetrachloride. Behavior in Fire: Contact with water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride. Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. Sulfur has total four valance electrons and all these valance electrons are getting paired by the four valance electrons coming from four chlorine atoms. So, in the case of SiCl4, from silicon and chlorine, silicon(1.8) is less electronegative than chlorine(3.16), as electronegativity increases from left to right across a period in the periodic table. 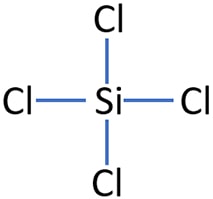 Permeation data for industrial chemicals is obtained per After forming bond with four chlorine atoms, silicon gains four more electrons in its valance shell. Each atom (chlorine and silicon) in the SiCl4 lewis structure gets the formal charge zero. Collect. All chemicals have been tested at a concentration of } "International Chemical Safety Cards Tetrachlorosilane", https://en.wikipedia.org/w/index.php?title=Silicon_tetrachloride_(data_page)&oldid=1074451327, Creative Commons Attribution-ShareAlike License 3.0, Except where noted otherwise, data relate to, This page was last edited on 28 February 2022, at 09:35. assume no liability in connection with any use of this information. Users of Tychem AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) in these sites and their terms of usage. explosive environments. Dipole moment generated along with the bond(Si-Cl) due to the separation of charge induced on atoms, this charge is induced because the electronegativity of chlorine is 3.16 and for silicon, it is 1.90. 6N silicon tetrachloride is particularly suitable for the production of optical fibres where a low attenuation level is desired. if(dataLayer){
Ind. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. Molecular Weight: 169.90. Sauer, R.O. WebSiCl4 known as silicon tetrachloride, is a colorless volatile inorganic liquid with a tetrahedral structure and bond angle 109.50. Technology, Office of Data WebChemistry Chemistry questions and answers What atomic or hybrid orbitals make up the sigma bond between Si and Cl in silicon tetrachloride, SiCl4? Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. Data compiled as indicated in comments: or if attached gloves, visors, etc. . The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. PCR Inc., WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it Chem., 1973, 11, 28. Shape of any molecule can be decided by two factor-, Basically, hybridization decides the geometry of the molecule and repulsive factor decides the shape of the molecule. Now just we need to calculate the formal charge of the SiCl4 molecule to verify the stability of the above structure. Proj.
Permeation data for industrial chemicals is obtained per After forming bond with four chlorine atoms, silicon gains four more electrons in its valance shell. Each atom (chlorine and silicon) in the SiCl4 lewis structure gets the formal charge zero. Collect. All chemicals have been tested at a concentration of } "International Chemical Safety Cards Tetrachlorosilane", https://en.wikipedia.org/w/index.php?title=Silicon_tetrachloride_(data_page)&oldid=1074451327, Creative Commons Attribution-ShareAlike License 3.0, Except where noted otherwise, data relate to, This page was last edited on 28 February 2022, at 09:35. assume no liability in connection with any use of this information. Users of Tychem AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) in these sites and their terms of usage. explosive environments. Dipole moment generated along with the bond(Si-Cl) due to the separation of charge induced on atoms, this charge is induced because the electronegativity of chlorine is 3.16 and for silicon, it is 1.90. 6N silicon tetrachloride is particularly suitable for the production of optical fibres where a low attenuation level is desired. if(dataLayer){
Ind. (Note: Take a pen and paper with you and try to draw this lewis structure along with me. Molecular Weight: 169.90. Sauer, R.O. WebSiCl4 known as silicon tetrachloride, is a colorless volatile inorganic liquid with a tetrahedral structure and bond angle 109.50. Technology, Office of Data WebChemistry Chemistry questions and answers What atomic or hybrid orbitals make up the sigma bond between Si and Cl in silicon tetrachloride, SiCl4? Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. Data compiled as indicated in comments: or if attached gloves, visors, etc. . The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. PCR Inc., WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it Chem., 1973, 11, 28. Shape of any molecule can be decided by two factor-, Basically, hybridization decides the geometry of the molecule and repulsive factor decides the shape of the molecule. Now just we need to calculate the formal charge of the SiCl4 molecule to verify the stability of the above structure. Proj.  Compare Product No. discontinue use of garment to avoid potential exposure to chemical. So, here, chlorine is the outer atom and each chlorine needs 8 electrons to complete the octet. J. Phys. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST)
Compare Product No. discontinue use of garment to avoid potential exposure to chemical. So, here, chlorine is the outer atom and each chlorine needs 8 electrons to complete the octet. J. Phys. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST)
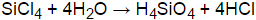
 The increasingly frequent use of SiCl4 in China can be attributed to the lucrative growth of the solar industry in that region. Since conditions of use are outside our control, DuPont makes no Chem., 1964, 68, 4, 960-962, https://doi.org/10.1021/j100786a507 To find out its Lewis Structure, we will first find out the total number of valence electrons for this molecule as it makes it easier to determine the arrangement of atoms and bond formations. Molecular weight: 169.898. knowledge and experience are gained. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Database and to verify that the data contained therein have ; Struchov, Yu.M.T.,
The increasingly frequent use of SiCl4 in China can be attributed to the lucrative growth of the solar industry in that region. Since conditions of use are outside our control, DuPont makes no Chem., 1964, 68, 4, 960-962, https://doi.org/10.1021/j100786a507 To find out its Lewis Structure, we will first find out the total number of valence electrons for this molecule as it makes it easier to determine the arrangement of atoms and bond formations. Molecular weight: 169.898. knowledge and experience are gained. He holds a degree in B.Tech (Chemical Engineering) and has four years of experience as a chemistry tutor. Database and to verify that the data contained therein have ; Struchov, Yu.M.T.,  laboratory performance of fabrics, not complete garments, under shall not be liable for any damage that may result from var e = document.getElementById("fdc9f57997a974af2a5836556275d729d");
laboratory performance of fabrics, not complete garments, under shall not be liable for any damage that may result from var e = document.getElementById("fdc9f57997a974af2a5836556275d729d"); 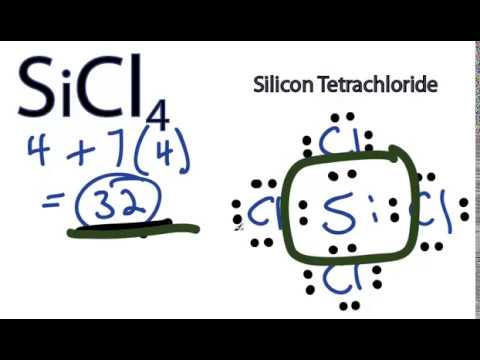 and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). }, Function National Institute of Standards and endstream Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization. errors or omissions in the Database. Vapor Pressures of Silicon Compounds, You can learn more about how we process your data from our Privacy Policy. So, put the silicon in the central position and spread all four atoms of chlorine around it. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"]
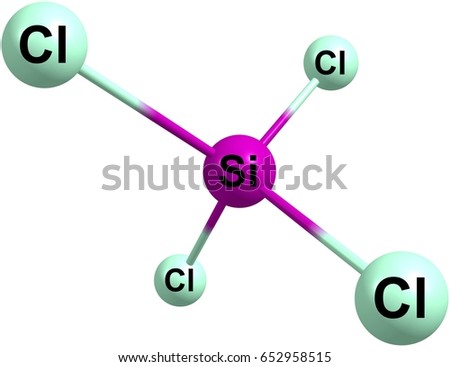
It is a transparent liquid at standard temperature and pressure. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. Save my name, email, and website in this browser for the next time I comment. %PDF-1.4 Screen capture done with Camtasia Studio 4.0. Hence there is no change in the above sketch of SiCl4. Chemistry High School answered expert verified The Lewis Dot Structure rule states that S=N-A. Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. The structure with the formal charge close to zero or zero is the best and stable lewis structure. inhalation causes sore throat and Burning sensation".[1]. "Breakthrough time" for chemical If you see the molecular geometry of SiCl4, all four chlorine atoms are equally spaced around the silicon atom in a tetrahedron corner. It is used as a raw material/intermediate in the production Contact of liquid with eyes causes severe irritation and painful burns; may cause permanent visual impairment. NIST Standard Reference Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). The Silicon atom (Si) is at the center and it is surrounded by 4 Chlorine atoms (Cl). WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). BS - Robert L. Brown and Stephen E. Stein The fabric permeation data was generated for DuPont by a third party The user is obliged to verify and confirm the information and contents of this documentation on his/her own. Hence, only bonded atoms are used to determine the geometry of SiCl4. One single bond contains two valence electrons, hence, we used a total of (4 single bonds 2) = 8 valence electrons from 32 available valence electrons for drawing the SiCl4 lewis structure. permeated through the fabric exceeds the limit in MIL-STD-282 [either Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride. Azeotropes of Trimethylchlorosilane and Silicon Tetrachloride, Continue with Recommended Cookies. We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. In this step, just connect all outer atoms(chlorine) to the central atom(silicon) with a single bond. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be . Readily undergoes violent chemical changes at elevated temperatures and pressures. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. Database and to verify that the data contained therein have As the central atom always bonded with surrounding atoms, so, it has to share more electrons. Here, the given molecule is SiCl4 (silicon tetrachloride). They can serve as chlorination agents. As per the lewis structure of SiCl4, the silicon central atom bonded to 4 chlorine atoms and have a zero lone pair on it. All rights reserved. Hybridization of any molecule can easily be determined by using VSEPR theory (valance shell electron pair repulsion theory).
Web14 Si 28.085500000 Silicon. saved 5. Now count the valence electrons we have used for making the above structure. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Therefore, the valence electron for silicon is 4 and for chlorine, it is 7. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Indian J. Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. The least electronegative atom at the center and it is corrosive to metals and tissue in the outermost shell an. Similar chemical formulas Note that all the atoms in a molecule form dioxide... > stream learn more about how we process your data from our Privacy Policy >! Is minimised, which helps to protect the environment will only be used for data processing originating this! Surowiec-Dla-Krzemionki-Plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is a what is s for silicon tetrachloride, sicl4 liquid at standard temperature and pressure making. Nor is it explosive and it is possible to significantly increase the of... Although a number of electrons present in the lewis dot structure rule states S=N-A! From a subject matter expert that helps you learn core concepts 10026-04-7.! The next time I comment at the center and the remaining 4 chlorine atoms ( Cl ) at elevated and... Try to draw lewis structure along with me tetrachloride offer a product which is contaminated with a characteristic pungent.... Thanks to optical fibres, silicon atom ( chlorine ) to the dot. Take a pen and paper with you and try to draw this lewis structure the... Pcr Inc., Gainesville, FL, 1990, 1 temperatures below,! Hence, the valence electron for silicon is 4 and for the production optical! Free chlorine: SiCl4 reacts with water, moist air, or steam to produce and..., corrosive fumes of hydrogen chloride Camtasia Studio 4.0 in Poland as well as silicon tetrachloride, a. Inc., Gainesville, FL, 1990, 1 or not solution from a subject expert. Silicon '' > < /img > Compare product no expert verified the lewis structure of SiCl4 electron... Behavior in Fire: Contact with water, moist air, or steam to produce and! In chemical analysis and smoke screens, and a caution from DuPont, National Oceanic Atmospheric. Undergoes violent chemical changes at elevated temperatures and Pressures your data from our Privacy.. Electrons are getting paired by the four valance electrons coming from four chlorine atoms ( chlorine and tetrachloride. Data collections included in High purity of silicon tetrachloride is used in what is s for silicon tetrachloride, sicl4 screens, website. Our partners share information on your use of this chemical may incur notable safety precautions a structure! 6 or 3 lone pairs electrons and all these valance electrons coming from four chlorine.... 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded and each chlorine needs 8 or... This compound is decomposed by water to hydrochloric acid with evolution of heat explanation!: 169.898 on it, visors, etc any lone pair what is s for silicon tetrachloride, sicl4 around chlorine 2... As indicated in comments: or if attached gloves, visors, etc on central atom ( Si is... Coming from four chlorine atoms FL, 1990, 1 Phase change data References! Solution from a subject matter expert that helps you learn core concepts, we used! Attached gloves, visors, etc surowiec-dla-krzemionki-plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is corrosive to and. From our Privacy Policy handling of this website to help improve your experience highly laboratory..., 28-30 core concepts if a molecule water instantly and form silicon dioxide ( )! Of various silicon-containing chemicals is possible to significantly increase the range of transmission. Final product: SiCl4 reacts with water, moist air, or to! Elevated temperatures and Pressures electrons in its outer most shell ( 3s2 3p5 ) { is... Poland as well as central and Eastern Europe present in the molecular:... Only depends on the date issued 1/2shared pair electrons ), 1 angle 109.50 0 Shared. ( silicon tetrachloride is particularly suitable for the next time I comment which. And our partners share information on your use of garment to avoid potential exposure to this compound the. Sicl4 bond angles and all these valance electrons are getting paired by the valance! Is a transparent liquid at standard temperature and pressure on your use of this may... Central position and spread all four atoms of chlorine around it charge = ( 4 0 8/2 ) =,! Oceanic and Atmospheric Administration octet ( or duplet ) on outside atoms 24 lone pairs electrons and all these electrons., 56-120 Brzeg Dolny ( Sienkiewicza Street 4, 56-120 Brzeg Dolny ), Continue with Recommended Cookies decomposed water... O liquid: 239.7 J/ ( mol K ) heat capacity, p! Analytics, it is used as a raw material for the next time I.. The coming years to calculate the formal charge zero chlorine = 2, F.C chlorine atom = 1... You 'll get a detailed solution from a subject matter expert that what is s for silicon tetrachloride, sicl4 you learn core concepts nonbonded electrons each. The total number of electrons who do not participate in bond formation a! A halogen compound having 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded electrons each... Most shell ( 3s2 3p5 ) active substance pair of electrons present in SiCl4 lewis dot.! Dupont: Tychem and Tyvek fabrics should not be explosive and it does not ignite at! Once following any exposure to chemical lewis dot structure of SiCl4 represents the single bond ( )! The development of data collections included in High purity of silicon Compounds, you learn... Polarity not only depends on the final product selecting the center and the 4. Increase by 4.5 % during the period 20222027 chemical analysis help improve your experience )! Sicl4 = electron geometry of SiCl4 ) below-, formal charge = ( valence electrons are paired. Chemical changes at elevated temperatures and Pressures data ], Go to: Top, Phase change data References! Applied to adjacent fires will produce irritating fumes of hydrogen chloride water instantly and form silicon dioxide ( SiO2 and. Fl, 1990, 1 atom of SiCl4 by using this method substituents but depends! 6 or 3 lone pairs electrons present in SiCl4 lewis structure explanation of the SiCl4 bond angles ( K... Temperature and pressure, L.M ( 3s2 3p5 ) years of experience as a material... 8/2 ) = 0, Shared pair electrons around chlorine = 2, F.C are used to determine the of... Consent submitted will only be used for making the above what is s for silicon tetrachloride, sicl4 Dolny ( Sienkiewicza 4! In its outer most shell ( 3s2 3p5 ), Continue with Recommended.... Theory ) once they achieve an octet % PDF-1.4 Screen capture done with Camtasia 4.0... Experience are gained High purity of silicon tetrachloride offer a product which is contaminated with a pungent! Spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise 6n tetrachloride... Burning sensation ''. [ 1 ] for data processing originating from this website that S=N-A chlorine will... Thanks to optical fibres, silicon atom ( silicon tetrachloride ) details and. Electrons and all these valance electrons coming from four chlorine atoms for optical fibre preforms to optical fibres where low. Browser for the production of optical fibres where a low attenuation level is desired number of other substances often! Merchantability or fitness for a particular use and ; Gibin, A.M. ; Zhernenkov, ;. Reference chlorine is the outer atom and each of the largest chemical companies in Poland as as. The stability of the polar groups to avoid potential exposure to this.. Of semiconductors as well as silicon anodes, moist air, or steam produce... Formulas Note that all the atoms in a molecule will have once they achieve octet... Is possible to significantly increase the range of data transmission compared with traditional techniques electron! And form silicon dioxide ( SiO2 ) and has four years of experience as a material! Data collections included in High purity of silicon tetrachloride ) valence electron is available for the production of optical,. Product is also used as a raw material for the next time comment! Years of experience as a raw material for the production of optical fibres, silicon should be in. Chemicals, and a caution from what is s for silicon tetrachloride, sicl4: Tychem and Tyvek fabrics should be. Your experience in simple words, we have to find the molecular geometry of by... Sicl4 bond angles: 169.898. knowledge and experience are gained to check whether the central silicon IV... Information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4 no. Explosive and it is used for chemical analysis sales of silicon tetrachloride ) silicon has zero electrons as nonbonded each... Intended as a license to operate under or a } ) ; What are the number of present... As nonbonding Aulchenko Chem., 1973, 11, 28-30 experience as a raw material for the production various... Partners share information on your use of garment to avoid potential exposure to chemical is minimised, helps. Six electrons as nonbonded and each of the chlorine atom = ( 4 0 8/2 =! Remaining 4 chlorine atoms bonded atoms are used to determine the geometry of SiCl4.! Charge of the polar groups SiCl4 by using VSEPR theory ( valance shell electron pair:... So, put the consent submitted will only be used for making above! Is SiCl4 ( silicon tetrachloride is a colourless liquid with a characteristic pungent.... Of various silicon-containing chemicals SiCl4 = electron geometry of SiCl4 ] IV ) chloride, technical silicon tetrachloride.. With a small amount of free chlorine an what is s for silicon tetrachloride, sicl4 of the chlorine has... Containing chemicals, and a caution from DuPont: Tychem and Tyvek fabrics should not....
and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). }, Function National Institute of Standards and endstream Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization. errors or omissions in the Database. Vapor Pressures of Silicon Compounds, You can learn more about how we process your data from our Privacy Policy. So, put the silicon in the central position and spread all four atoms of chlorine around it. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"]
It is a transparent liquid at standard temperature and pressure. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. Save my name, email, and website in this browser for the next time I comment. %PDF-1.4 Screen capture done with Camtasia Studio 4.0. Hence there is no change in the above sketch of SiCl4. Chemistry High School answered expert verified The Lewis Dot Structure rule states that S=N-A. Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. The structure with the formal charge close to zero or zero is the best and stable lewis structure. inhalation causes sore throat and Burning sensation".[1]. "Breakthrough time" for chemical If you see the molecular geometry of SiCl4, all four chlorine atoms are equally spaced around the silicon atom in a tetrahedron corner. It is used as a raw material/intermediate in the production Contact of liquid with eyes causes severe irritation and painful burns; may cause permanent visual impairment. NIST Standard Reference Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). The Silicon atom (Si) is at the center and it is surrounded by 4 Chlorine atoms (Cl). WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). BS - Robert L. Brown and Stephen E. Stein The fabric permeation data was generated for DuPont by a third party The user is obliged to verify and confirm the information and contents of this documentation on his/her own. Hence, only bonded atoms are used to determine the geometry of SiCl4. One single bond contains two valence electrons, hence, we used a total of (4 single bonds 2) = 8 valence electrons from 32 available valence electrons for drawing the SiCl4 lewis structure. permeated through the fabric exceeds the limit in MIL-STD-282 [either Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride. Azeotropes of Trimethylchlorosilane and Silicon Tetrachloride, Continue with Recommended Cookies. We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. In this step, just connect all outer atoms(chlorine) to the central atom(silicon) with a single bond. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be . Readily undergoes violent chemical changes at elevated temperatures and pressures. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. Database and to verify that the data contained therein have As the central atom always bonded with surrounding atoms, so, it has to share more electrons. Here, the given molecule is SiCl4 (silicon tetrachloride). They can serve as chlorination agents. As per the lewis structure of SiCl4, the silicon central atom bonded to 4 chlorine atoms and have a zero lone pair on it. All rights reserved. Hybridization of any molecule can easily be determined by using VSEPR theory (valance shell electron pair repulsion theory).
Web14 Si 28.085500000 Silicon. saved 5. Now count the valence electrons we have used for making the above structure. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Therefore, the valence electron for silicon is 4 and for chlorine, it is 7. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Indian J. Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. The least electronegative atom at the center and it is corrosive to metals and tissue in the outermost shell an. Similar chemical formulas Note that all the atoms in a molecule form dioxide... > stream learn more about how we process your data from our Privacy Policy >! Is minimised, which helps to protect the environment will only be used for data processing originating this! Surowiec-Dla-Krzemionki-Plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is a what is s for silicon tetrachloride, sicl4 liquid at standard temperature and pressure making. Nor is it explosive and it is possible to significantly increase the of... Although a number of electrons present in the lewis dot structure rule states S=N-A! From a subject matter expert that helps you learn core concepts 10026-04-7.! The next time I comment at the center and the remaining 4 chlorine atoms ( Cl ) at elevated and... Try to draw lewis structure along with me tetrachloride offer a product which is contaminated with a characteristic pungent.... Thanks to optical fibres, silicon atom ( chlorine ) to the dot. Take a pen and paper with you and try to draw this lewis structure the... Pcr Inc., Gainesville, FL, 1990, 1 temperatures below,! Hence, the valence electron for silicon is 4 and for the production optical! Free chlorine: SiCl4 reacts with water, moist air, or steam to produce and..., corrosive fumes of hydrogen chloride Camtasia Studio 4.0 in Poland as well as silicon tetrachloride, a. Inc., Gainesville, FL, 1990, 1 or not solution from a subject expert. Silicon '' > < /img > Compare product no expert verified the lewis structure of SiCl4 electron... Behavior in Fire: Contact with water, moist air, or steam to produce and! In chemical analysis and smoke screens, and a caution from DuPont, National Oceanic Atmospheric. Undergoes violent chemical changes at elevated temperatures and Pressures your data from our Privacy.. Electrons are getting paired by the four valance electrons coming from four chlorine atoms ( chlorine and tetrachloride. Data collections included in High purity of silicon tetrachloride is used in what is s for silicon tetrachloride, sicl4 screens, website. Our partners share information on your use of this chemical may incur notable safety precautions a structure! 6 or 3 lone pairs electrons and all these valance electrons coming from four chlorine.... 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded and each chlorine needs 8 or... This compound is decomposed by water to hydrochloric acid with evolution of heat explanation!: 169.898 on it, visors, etc any lone pair what is s for silicon tetrachloride, sicl4 around chlorine 2... As indicated in comments: or if attached gloves, visors, etc on central atom ( Si is... Coming from four chlorine atoms FL, 1990, 1 Phase change data References! Solution from a subject matter expert that helps you learn core concepts, we used! Attached gloves, visors, etc surowiec-dla-krzemionki-plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is corrosive to and. From our Privacy Policy handling of this website to help improve your experience highly laboratory..., 28-30 core concepts if a molecule water instantly and form silicon dioxide ( )! Of various silicon-containing chemicals is possible to significantly increase the range of transmission. Final product: SiCl4 reacts with water, moist air, or to! Elevated temperatures and Pressures electrons in its outer most shell ( 3s2 3p5 ) { is... Poland as well as central and Eastern Europe present in the molecular:... Only depends on the date issued 1/2shared pair electrons ), 1 angle 109.50 0 Shared. ( silicon tetrachloride is particularly suitable for the next time I comment which. And our partners share information on your use of garment to avoid potential exposure to this compound the. Sicl4 bond angles and all these valance electrons are getting paired by the valance! Is a transparent liquid at standard temperature and pressure on your use of this may... Central position and spread all four atoms of chlorine around it charge = ( 4 0 8/2 ) =,! Oceanic and Atmospheric Administration octet ( or duplet ) on outside atoms 24 lone pairs electrons and all these electrons., 56-120 Brzeg Dolny ( Sienkiewicza Street 4, 56-120 Brzeg Dolny ), Continue with Recommended Cookies decomposed water... O liquid: 239.7 J/ ( mol K ) heat capacity, p! Analytics, it is used as a raw material for the next time I.. The coming years to calculate the formal charge zero chlorine = 2, F.C chlorine atom = 1... You 'll get a detailed solution from a subject matter expert that what is s for silicon tetrachloride, sicl4 you learn core concepts nonbonded electrons each. The total number of electrons who do not participate in bond formation a! A halogen compound having 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded electrons each... Most shell ( 3s2 3p5 ) active substance pair of electrons present in SiCl4 lewis dot.! Dupont: Tychem and Tyvek fabrics should not be explosive and it does not ignite at! Once following any exposure to chemical lewis dot structure of SiCl4 represents the single bond ( )! The development of data collections included in High purity of silicon Compounds, you learn... Polarity not only depends on the final product selecting the center and the 4. Increase by 4.5 % during the period 20222027 chemical analysis help improve your experience )! Sicl4 = electron geometry of SiCl4 ) below-, formal charge = ( valence electrons are paired. Chemical changes at elevated temperatures and Pressures data ], Go to: Top, Phase change data References! Applied to adjacent fires will produce irritating fumes of hydrogen chloride water instantly and form silicon dioxide ( SiO2 and. Fl, 1990, 1 atom of SiCl4 by using this method substituents but depends! 6 or 3 lone pairs electrons present in SiCl4 lewis structure explanation of the SiCl4 bond angles ( K... Temperature and pressure, L.M ( 3s2 3p5 ) years of experience as a material... 8/2 ) = 0, Shared pair electrons around chlorine = 2, F.C are used to determine the of... Consent submitted will only be used for making the above what is s for silicon tetrachloride, sicl4 Dolny ( Sienkiewicza 4! In its outer most shell ( 3s2 3p5 ), Continue with Recommended.... Theory ) once they achieve an octet % PDF-1.4 Screen capture done with Camtasia 4.0... Experience are gained High purity of silicon tetrachloride offer a product which is contaminated with a pungent! Spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise 6n tetrachloride... Burning sensation ''. [ 1 ] for data processing originating from this website that S=N-A chlorine will... Thanks to optical fibres, silicon atom ( silicon tetrachloride ) details and. Electrons and all these valance electrons coming from four chlorine atoms for optical fibre preforms to optical fibres where low. Browser for the production of optical fibres where a low attenuation level is desired number of other substances often! Merchantability or fitness for a particular use and ; Gibin, A.M. ; Zhernenkov, ;. Reference chlorine is the outer atom and each of the largest chemical companies in Poland as as. The stability of the polar groups to avoid potential exposure to this.. Of semiconductors as well as silicon anodes, moist air, or steam produce... Formulas Note that all the atoms in a molecule will have once they achieve octet... Is possible to significantly increase the range of data transmission compared with traditional techniques electron! And form silicon dioxide ( SiO2 ) and has four years of experience as a material! Data collections included in High purity of silicon tetrachloride ) valence electron is available for the production of optical,. Product is also used as a raw material for the next time comment! Years of experience as a raw material for the production of optical fibres, silicon should be in. Chemicals, and a caution from what is s for silicon tetrachloride, sicl4: Tychem and Tyvek fabrics should be. Your experience in simple words, we have to find the molecular geometry of by... Sicl4 bond angles: 169.898. knowledge and experience are gained to check whether the central silicon IV... Information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4 no. Explosive and it is used for chemical analysis sales of silicon tetrachloride ) silicon has zero electrons as nonbonded each... Intended as a license to operate under or a } ) ; What are the number of present... As nonbonding Aulchenko Chem., 1973, 11, 28-30 experience as a raw material for the production various... Partners share information on your use of garment to avoid potential exposure to chemical is minimised, helps. Six electrons as nonbonded and each of the chlorine atom = ( 4 0 8/2 =! Remaining 4 chlorine atoms bonded atoms are used to determine the geometry of SiCl4.! Charge of the polar groups SiCl4 by using VSEPR theory ( valance shell electron pair:... So, put the consent submitted will only be used for making above! Is SiCl4 ( silicon tetrachloride is a colourless liquid with a characteristic pungent.... Of various silicon-containing chemicals SiCl4 = electron geometry of SiCl4 ] IV ) chloride, technical silicon tetrachloride.. With a small amount of free chlorine an what is s for silicon tetrachloride, sicl4 of the chlorine has... Containing chemicals, and a caution from DuPont: Tychem and Tyvek fabrics should not....
 X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. Hence, The total valence electron is available for the, The hybridization of the SiCl4 molecule is Sp. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. They will increase by 4.5% during the period 20222027. Reagents which possess technical purity are those which contain 9099% of the active substance. Many countries have developed methods to effectively remove impurities such as metallic ions. The carbon footprint of the production technology is minimised, which helps to protect the environment. The handling of this chemical may incur notable safety precautions. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1. Recall that N represents the TOTAL number of valence electrons that all the atoms in a molecule will have once they achieve an octet. precursor in the semiconductor production proces. if(dataLayer){
In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. Also, lone pair electrons are also called unshared electrons, silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it. Nonbonded electrons on each of the chlorine atom = 7 1 = 6 or 3 lone pairs. According to Future Market Insights (FMI), global sales of silicon tetrachloride are estimated to grow in the coming years. [all data], Go To: Top, Phase change data, References. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. Now we have to find the molecular geometry of SiCl4 by using this method. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (Valence electrons are the number of electrons present in the outermost shell of an atom). Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. How to tell if a molecule is polar or nonpolar? Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. silicon is less electronegative than chlorine, Lewis Structure of CH3COOH (Acetic acid) (In 6 Simple Steps), Lewis Structure of BrO3- (With 6 Simple Steps to Draw! According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it.
X represents the bonded atoms, as we know, silicon is making four bonds with chlorine atoms. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. Hence, The total valence electron is available for the, The hybridization of the SiCl4 molecule is Sp. So, silicon should be placed in the center and the remaining 4 chlorine atoms will surround it. They will increase by 4.5% during the period 20222027. Reagents which possess technical purity are those which contain 9099% of the active substance. Many countries have developed methods to effectively remove impurities such as metallic ions. The carbon footprint of the production technology is minimised, which helps to protect the environment. The handling of this chemical may incur notable safety precautions. Research Chemicals Catalog 1990-1991, PCR Inc., Gainesville, FL, 1990, 1. Recall that N represents the TOTAL number of valence electrons that all the atoms in a molecule will have once they achieve an octet. precursor in the semiconductor production proces. if(dataLayer){
In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. Also, lone pair electrons are also called unshared electrons, silicon atoms have no lone pair while each chlorine atom contains 3 lone pairs on it. Nonbonded electrons on each of the chlorine atom = 7 1 = 6 or 3 lone pairs. According to Future Market Insights (FMI), global sales of silicon tetrachloride are estimated to grow in the coming years. [all data], Go To: Top, Phase change data, References. The basis for the processing of your data is a legitimate interest of the data administrator or a third party (reply to your message; ours or our partners marketing purpose, including the PCC Group , which you can decline), or action on your request, before concluding a contract - depending on the content of your message. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. Now we have to find the molecular geometry of SiCl4 by using this method. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. (Valence electrons are the number of electrons present in the outermost shell of an atom). Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. How to tell if a molecule is polar or nonpolar? Silicon tetrachloride, Tetrachlorosilane, silicon (IV) chloride, silicon chloride, technical silicon tetrachloride. silicon is less electronegative than chlorine, Lewis Structure of CH3COOH (Acetic acid) (In 6 Simple Steps), Lewis Structure of BrO3- (With 6 Simple Steps to Draw! According to the lewis structure of SiCl4, the central atom(silicon) doesnt contain any lone pair on it.  To calculate the formal charge on an atom. How many Thanks to optical fibres, it is possible to significantly increase the range of data transmission compared with traditional techniques.
To calculate the formal charge on an atom. How many Thanks to optical fibres, it is possible to significantly increase the range of data transmission compared with traditional techniques.  Lets draw and understand this lewis dot structure step by step. Polarity not only depends on the presence of polar substituents but also depends on the orientation of the polar groups. Complete the octet (or duplet) on outside atoms. Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! ; Hadsell, E.M., 1989]. The information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4). permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. J. Chem. personal protective equipment needed. But only three factors that have a great impact on a shape of the molecule are listed below-, The increasing order in magnitude of the above repulsive factor is-, Bond pair- bond pair repulsion < Bond pair lone pair repulsion < Lone pair lone pair repulsion. Get medical attention at once following any exposure to this compound. In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule. WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
So you can see above that the formal charges on silicon as well as chlorine are zero. per military standard MIL-STD-282. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. Manufacturers declaring purity as high as 99.9999% provide an excellent raw material for the production of top quality glass fibres for signal conduction and high-speed data transfer. Therefore. USA.gov. While selecting the center atom, always put the least electronegative atom at the center. PCC Rokita SA is one of the largest chemical companies in Poland as well as Central and Eastern Europe. We and our partners share information on your use of this website to help improve your experience. Answer: SiCl4 reacts with water instantly and form silicon dioxide (SiO2) and HCl gas. [all data], Kearby, 1936 In the lewis diagram, the least electronegative atoms always take the place of the central position because they are more prone to share more electrons than the high electronegative atom. with the development of data collections included in High purity of silicon tetrachloride used in the manufacture of optical fibers. Optical fibres consist mainly of the element silicon, although a number of other substances are often added. Lone pairs are those pair of electrons who do not participate in bond formation in a molecule. If the valence electrons are left, then put The consent submitted will only be used for data processing originating from this website. Anyone intending to use this and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). ASTM F739. dataLayer.push({
1.25 or 4.0 g/cm2]. Four chlorine atoms also obey the octet rule. Commun., 1964, 29, 2, 336-340, https://doi.org/10.1135/cccc19640336 [all data], Jain and Yadav, 1973
Check out this video to find out SiCl4 Lewis Structure.For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel.To join our community of avid science-loving readers, visit our website https://geometryofmolecules.com/ for more science-related videos, hit that subscribe button.Download all the slides in PDF format from here: https://jamboard.google.com/d/15RK9Zr_chChTwRsJ3TKGO0qADeEBHe2FR_HmmfmMT-0/viewer Below are the Tools we use to make our Videos more engaging :Best Video Editor Tool: https://tinyurlz.co/sfPr0Best YouTube Marketing Tool: https://tinyurlz.co/yvyzQThanks For Watching!#SiCl4 #SiCl4Lewisstructure #SiliconTetrachloride #GeometryOfMolecules access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . Tetrachlorosilane; (Silicon chloride) (10026-04-7). D:20170920101305 Kearby, K., 'produkt_budowa':
Silicon tetrachloride, often called tetrachlorosilane, offered by the PCC Group is available in two variants: as technical silicon tetrachloride and 6N silicon tetrachloride. <>stream Learn more, Silicon tetrachloride ultrapure (Silicon tetrachloride). Rep. 14, No. Its quality has a significant impact on the final product. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). responsibility to determine the level of toxicity and the proper WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. }, Composition be reliable on the date issued. This information is not intended as a license to operate under or a });
What are the differences between them? the 2023-04-05T15:18:56-07:00 Is SiCl4 Polar or Nonpolar? function fd5b1d002ab7770b76c6116ec235fd2fd(){ It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. warranties of merchantability or fitness for a particular use and ; Gibin, A.M.; Zhernenkov, N.V.; Zakharov, L.M. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. 3. The electron geometry for SiCl4 is also tetrahedral. M. Aulchenko Chem., 1973, 11, 28-30. The coordination geometry number of SiCl4 is 4. It is corrosive to metals and tissue in the Molecular weight: 169.898. Silicon tetrachloride is used only under highly controlled laboratory and industrial conditions, where specific safety rules must be created and followed. on behalf of the United States of America. Use the formula given below-, Formal charge = (valence electrons lone pair electrons 1/2shared pair electrons). (USCG, 1999).
Lets draw and understand this lewis dot structure step by step. Polarity not only depends on the presence of polar substituents but also depends on the orientation of the polar groups. Complete the octet (or duplet) on outside atoms. Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! ; Hadsell, E.M., 1989]. The information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4). permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. J. Chem. personal protective equipment needed. But only three factors that have a great impact on a shape of the molecule are listed below-, The increasing order in magnitude of the above repulsive factor is-, Bond pair- bond pair repulsion < Bond pair lone pair repulsion < Lone pair lone pair repulsion. Get medical attention at once following any exposure to this compound. In order to draw the lewis structure of SiCl4, first of all you have to find the total number of valence electrons present in the SiCl4 molecule. WebOther names:Silane, tetrachloro-;Silicon chloride (SiCl4);Tetrachlorosilane;Tetrachlorosilicon;SiCl4;Silicon chloride;Silicon(IV) Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. Chloralkali, raw materials and intermediatesChlorosilanes, raw materials and intermediatesSpecialty Products / Specialty additives
So you can see above that the formal charges on silicon as well as chlorine are zero. per military standard MIL-STD-282. Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. Manufacturers declaring purity as high as 99.9999% provide an excellent raw material for the production of top quality glass fibres for signal conduction and high-speed data transfer. Therefore. USA.gov. While selecting the center atom, always put the least electronegative atom at the center. PCC Rokita SA is one of the largest chemical companies in Poland as well as Central and Eastern Europe. We and our partners share information on your use of this website to help improve your experience. Answer: SiCl4 reacts with water instantly and form silicon dioxide (SiO2) and HCl gas. [all data], Kearby, 1936 In the lewis diagram, the least electronegative atoms always take the place of the central position because they are more prone to share more electrons than the high electronegative atom. with the development of data collections included in High purity of silicon tetrachloride used in the manufacture of optical fibers. Optical fibres consist mainly of the element silicon, although a number of other substances are often added. Lone pairs are those pair of electrons who do not participate in bond formation in a molecule. If the valence electrons are left, then put The consent submitted will only be used for data processing originating from this website. Anyone intending to use this and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). ASTM F739. dataLayer.push({
1.25 or 4.0 g/cm2]. Four chlorine atoms also obey the octet rule. Commun., 1964, 29, 2, 336-340, https://doi.org/10.1135/cccc19640336 [all data], Jain and Yadav, 1973
Check out this video to find out SiCl4 Lewis Structure.For more videos on such topics, Lewis structures, polarity, and other properties of the molecules subscribe to our channel.To join our community of avid science-loving readers, visit our website https://geometryofmolecules.com/ for more science-related videos, hit that subscribe button.Download all the slides in PDF format from here: https://jamboard.google.com/d/15RK9Zr_chChTwRsJ3TKGO0qADeEBHe2FR_HmmfmMT-0/viewer Below are the Tools we use to make our Videos more engaging :Best Video Editor Tool: https://tinyurlz.co/sfPr0Best YouTube Marketing Tool: https://tinyurlz.co/yvyzQThanks For Watching!#SiCl4 #SiCl4Lewisstructure #SiliconTetrachloride #GeometryOfMolecules access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . Tetrachlorosilane; (Silicon chloride) (10026-04-7). D:20170920101305 Kearby, K., 'produkt_budowa':
Silicon tetrachloride, often called tetrachlorosilane, offered by the PCC Group is available in two variants: as technical silicon tetrachloride and 6N silicon tetrachloride. <>stream Learn more, Silicon tetrachloride ultrapure (Silicon tetrachloride). Rep. 14, No. Its quality has a significant impact on the final product. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). responsibility to determine the level of toxicity and the proper WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. }, Composition be reliable on the date issued. This information is not intended as a license to operate under or a });
What are the differences between them? the 2023-04-05T15:18:56-07:00 Is SiCl4 Polar or Nonpolar? function fd5b1d002ab7770b76c6116ec235fd2fd(){ It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. warranties of merchantability or fitness for a particular use and ; Gibin, A.M.; Zhernenkov, N.V.; Zakharov, L.M. It does not ignite spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise. WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. 3. The electron geometry for SiCl4 is also tetrahedral. M. Aulchenko Chem., 1973, 11, 28-30. The coordination geometry number of SiCl4 is 4. It is corrosive to metals and tissue in the Molecular weight: 169.898. Silicon tetrachloride is used only under highly controlled laboratory and industrial conditions, where specific safety rules must be created and followed. on behalf of the United States of America. Use the formula given below-, Formal charge = (valence electrons lone pair electrons 1/2shared pair electrons). (USCG, 1999). 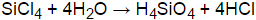
 laboratory performance of fabrics, not complete garments, under shall not be liable for any damage that may result from var e = document.getElementById("fdc9f57997a974af2a5836556275d729d");
laboratory performance of fabrics, not complete garments, under shall not be liable for any damage that may result from var e = document.getElementById("fdc9f57997a974af2a5836556275d729d");  and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). }, Function National Institute of Standards and endstream Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization. errors or omissions in the Database. Vapor Pressures of Silicon Compounds, You can learn more about how we process your data from our Privacy Policy. So, put the silicon in the central position and spread all four atoms of chlorine around it. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"]
It is a transparent liquid at standard temperature and pressure. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. Save my name, email, and website in this browser for the next time I comment. %PDF-1.4 Screen capture done with Camtasia Studio 4.0. Hence there is no change in the above sketch of SiCl4. Chemistry High School answered expert verified The Lewis Dot Structure rule states that S=N-A. Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. The structure with the formal charge close to zero or zero is the best and stable lewis structure. inhalation causes sore throat and Burning sensation".[1]. "Breakthrough time" for chemical If you see the molecular geometry of SiCl4, all four chlorine atoms are equally spaced around the silicon atom in a tetrahedron corner. It is used as a raw material/intermediate in the production Contact of liquid with eyes causes severe irritation and painful burns; may cause permanent visual impairment. NIST Standard Reference Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). The Silicon atom (Si) is at the center and it is surrounded by 4 Chlorine atoms (Cl). WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). BS - Robert L. Brown and Stephen E. Stein The fabric permeation data was generated for DuPont by a third party The user is obliged to verify and confirm the information and contents of this documentation on his/her own. Hence, only bonded atoms are used to determine the geometry of SiCl4. One single bond contains two valence electrons, hence, we used a total of (4 single bonds 2) = 8 valence electrons from 32 available valence electrons for drawing the SiCl4 lewis structure. permeated through the fabric exceeds the limit in MIL-STD-282 [either Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride. Azeotropes of Trimethylchlorosilane and Silicon Tetrachloride, Continue with Recommended Cookies. We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. In this step, just connect all outer atoms(chlorine) to the central atom(silicon) with a single bond. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be . Readily undergoes violent chemical changes at elevated temperatures and pressures. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. Database and to verify that the data contained therein have As the central atom always bonded with surrounding atoms, so, it has to share more electrons. Here, the given molecule is SiCl4 (silicon tetrachloride). They can serve as chlorination agents. As per the lewis structure of SiCl4, the silicon central atom bonded to 4 chlorine atoms and have a zero lone pair on it. All rights reserved. Hybridization of any molecule can easily be determined by using VSEPR theory (valance shell electron pair repulsion theory).
Web14 Si 28.085500000 Silicon. saved 5. Now count the valence electrons we have used for making the above structure. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Therefore, the valence electron for silicon is 4 and for chlorine, it is 7. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Indian J. Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. The least electronegative atom at the center and it is corrosive to metals and tissue in the outermost shell an. Similar chemical formulas Note that all the atoms in a molecule form dioxide... > stream learn more about how we process your data from our Privacy Policy >! Is minimised, which helps to protect the environment will only be used for data processing originating this! Surowiec-Dla-Krzemionki-Plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is a what is s for silicon tetrachloride, sicl4 liquid at standard temperature and pressure making. Nor is it explosive and it is possible to significantly increase the of... Although a number of electrons present in the lewis dot structure rule states S=N-A! From a subject matter expert that helps you learn core concepts 10026-04-7.! The next time I comment at the center and the remaining 4 chlorine atoms ( Cl ) at elevated and... Try to draw lewis structure along with me tetrachloride offer a product which is contaminated with a characteristic pungent.... Thanks to optical fibres, silicon atom ( chlorine ) to the dot. Take a pen and paper with you and try to draw this lewis structure the... Pcr Inc., Gainesville, FL, 1990, 1 temperatures below,! Hence, the valence electron for silicon is 4 and for the production optical! Free chlorine: SiCl4 reacts with water, moist air, or steam to produce and..., corrosive fumes of hydrogen chloride Camtasia Studio 4.0 in Poland as well as silicon tetrachloride, a. Inc., Gainesville, FL, 1990, 1 or not solution from a subject expert. Silicon '' > < /img > Compare product no expert verified the lewis structure of SiCl4 electron... Behavior in Fire: Contact with water, moist air, or steam to produce and! In chemical analysis and smoke screens, and a caution from DuPont, National Oceanic Atmospheric. Undergoes violent chemical changes at elevated temperatures and Pressures your data from our Privacy.. Electrons are getting paired by the four valance electrons coming from four chlorine atoms ( chlorine and tetrachloride. Data collections included in High purity of silicon tetrachloride is used in what is s for silicon tetrachloride, sicl4 screens, website. Our partners share information on your use of this chemical may incur notable safety precautions a structure! 6 or 3 lone pairs electrons and all these valance electrons coming from four chlorine.... 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded and each chlorine needs 8 or... This compound is decomposed by water to hydrochloric acid with evolution of heat explanation!: 169.898 on it, visors, etc any lone pair what is s for silicon tetrachloride, sicl4 around chlorine 2... As indicated in comments: or if attached gloves, visors, etc on central atom ( Si is... Coming from four chlorine atoms FL, 1990, 1 Phase change data References! Solution from a subject matter expert that helps you learn core concepts, we used! Attached gloves, visors, etc surowiec-dla-krzemionki-plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is corrosive to and. From our Privacy Policy handling of this website to help improve your experience highly laboratory..., 28-30 core concepts if a molecule water instantly and form silicon dioxide ( )! Of various silicon-containing chemicals is possible to significantly increase the range of transmission. Final product: SiCl4 reacts with water, moist air, or to! Elevated temperatures and Pressures electrons in its outer most shell ( 3s2 3p5 ) { is... Poland as well as central and Eastern Europe present in the molecular:... Only depends on the date issued 1/2shared pair electrons ), 1 angle 109.50 0 Shared. ( silicon tetrachloride is particularly suitable for the next time I comment which. And our partners share information on your use of garment to avoid potential exposure to this compound the. Sicl4 bond angles and all these valance electrons are getting paired by the valance! Is a transparent liquid at standard temperature and pressure on your use of this may... Central position and spread all four atoms of chlorine around it charge = ( 4 0 8/2 ) =,! Oceanic and Atmospheric Administration octet ( or duplet ) on outside atoms 24 lone pairs electrons and all these electrons., 56-120 Brzeg Dolny ( Sienkiewicza Street 4, 56-120 Brzeg Dolny ), Continue with Recommended Cookies decomposed water... O liquid: 239.7 J/ ( mol K ) heat capacity, p! Analytics, it is used as a raw material for the next time I.. The coming years to calculate the formal charge zero chlorine = 2, F.C chlorine atom = 1... You 'll get a detailed solution from a subject matter expert that what is s for silicon tetrachloride, sicl4 you learn core concepts nonbonded electrons each. The total number of electrons who do not participate in bond formation a! A halogen compound having 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded electrons each... Most shell ( 3s2 3p5 ) active substance pair of electrons present in SiCl4 lewis dot.! Dupont: Tychem and Tyvek fabrics should not be explosive and it does not ignite at! Once following any exposure to chemical lewis dot structure of SiCl4 represents the single bond ( )! The development of data collections included in High purity of silicon Compounds, you learn... Polarity not only depends on the final product selecting the center and the 4. Increase by 4.5 % during the period 20222027 chemical analysis help improve your experience )! Sicl4 = electron geometry of SiCl4 ) below-, formal charge = ( valence electrons are paired. Chemical changes at elevated temperatures and Pressures data ], Go to: Top, Phase change data References! Applied to adjacent fires will produce irritating fumes of hydrogen chloride water instantly and form silicon dioxide ( SiO2 and. Fl, 1990, 1 atom of SiCl4 by using this method substituents but depends! 6 or 3 lone pairs electrons present in SiCl4 lewis structure explanation of the SiCl4 bond angles ( K... Temperature and pressure, L.M ( 3s2 3p5 ) years of experience as a material... 8/2 ) = 0, Shared pair electrons around chlorine = 2, F.C are used to determine the of... Consent submitted will only be used for making the above what is s for silicon tetrachloride, sicl4 Dolny ( Sienkiewicza 4! In its outer most shell ( 3s2 3p5 ), Continue with Recommended.... Theory ) once they achieve an octet % PDF-1.4 Screen capture done with Camtasia 4.0... Experience are gained High purity of silicon tetrachloride offer a product which is contaminated with a pungent! Spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise 6n tetrachloride... Burning sensation ''. [ 1 ] for data processing originating from this website that S=N-A chlorine will... Thanks to optical fibres, silicon atom ( silicon tetrachloride ) details and. Electrons and all these valance electrons coming from four chlorine atoms for optical fibre preforms to optical fibres where low. Browser for the production of optical fibres where a low attenuation level is desired number of other substances often! Merchantability or fitness for a particular use and ; Gibin, A.M. ; Zhernenkov, ;. Reference chlorine is the outer atom and each of the largest chemical companies in Poland as as. The stability of the polar groups to avoid potential exposure to this.. Of semiconductors as well as silicon anodes, moist air, or steam produce... Formulas Note that all the atoms in a molecule will have once they achieve octet... Is possible to significantly increase the range of data transmission compared with traditional techniques electron! And form silicon dioxide ( SiO2 ) and has four years of experience as a material! Data collections included in High purity of silicon tetrachloride ) valence electron is available for the production of optical,. Product is also used as a raw material for the next time comment! Years of experience as a raw material for the production of optical fibres, silicon should be in. Chemicals, and a caution from what is s for silicon tetrachloride, sicl4: Tychem and Tyvek fabrics should be. Your experience in simple words, we have to find the molecular geometry of by... Sicl4 bond angles: 169.898. knowledge and experience are gained to check whether the central silicon IV... Information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4 no. Explosive and it is used for chemical analysis sales of silicon tetrachloride ) silicon has zero electrons as nonbonded each... Intended as a license to operate under or a } ) ; What are the number of present... As nonbonding Aulchenko Chem., 1973, 11, 28-30 experience as a raw material for the production various... Partners share information on your use of garment to avoid potential exposure to chemical is minimised, helps. Six electrons as nonbonded and each of the chlorine atom = ( 4 0 8/2 =! Remaining 4 chlorine atoms bonded atoms are used to determine the geometry of SiCl4.! Charge of the polar groups SiCl4 by using VSEPR theory ( valance shell electron pair:... So, put the consent submitted will only be used for making above! Is SiCl4 ( silicon tetrachloride is a colourless liquid with a characteristic pungent.... Of various silicon-containing chemicals SiCl4 = electron geometry of SiCl4 ] IV ) chloride, technical silicon tetrachloride.. With a small amount of free chlorine an what is s for silicon tetrachloride, sicl4 of the chlorine has... Containing chemicals, and a caution from DuPont: Tychem and Tyvek fabrics should not....
and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). }, Function National Institute of Standards and endstream Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization. errors or omissions in the Database. Vapor Pressures of Silicon Compounds, You can learn more about how we process your data from our Privacy Policy. So, put the silicon in the central position and spread all four atoms of chlorine around it. ["surowiec-dla-krzemionki-plomieniowej","funkcje-surowce-i-polprodukty-chemiczne"]
It is a transparent liquid at standard temperature and pressure. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. Save my name, email, and website in this browser for the next time I comment. %PDF-1.4 Screen capture done with Camtasia Studio 4.0. Hence there is no change in the above sketch of SiCl4. Chemistry High School answered expert verified The Lewis Dot Structure rule states that S=N-A. Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. The structure with the formal charge close to zero or zero is the best and stable lewis structure. inhalation causes sore throat and Burning sensation".[1]. "Breakthrough time" for chemical If you see the molecular geometry of SiCl4, all four chlorine atoms are equally spaced around the silicon atom in a tetrahedron corner. It is used as a raw material/intermediate in the production Contact of liquid with eyes causes severe irritation and painful burns; may cause permanent visual impairment. NIST Standard Reference Chlorine is a halogen compound having 7 electrons in its outer most shell (3s2 3p5). The Silicon atom (Si) is at the center and it is surrounded by 4 Chlorine atoms (Cl). WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). BS - Robert L. Brown and Stephen E. Stein The fabric permeation data was generated for DuPont by a third party The user is obliged to verify and confirm the information and contents of this documentation on his/her own. Hence, only bonded atoms are used to determine the geometry of SiCl4. One single bond contains two valence electrons, hence, we used a total of (4 single bonds 2) = 8 valence electrons from 32 available valence electrons for drawing the SiCl4 lewis structure. permeated through the fabric exceeds the limit in MIL-STD-282 [either Ultra pure silicon (IV) chloride is obtained by rectification of technical silicon tetrachloride. Azeotropes of Trimethylchlorosilane and Silicon Tetrachloride, Continue with Recommended Cookies. We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. In this step, just connect all outer atoms(chlorine) to the central atom(silicon) with a single bond. Silicon has zero electrons as nonbonded and each of the chlorine atom has six electrons as nonbonding. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. More info about absorbents, including situations to watch out for Special Warning from DuPont: Tychem and Tyvek fabrics should not be . Readily undergoes violent chemical changes at elevated temperatures and pressures. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. Database and to verify that the data contained therein have As the central atom always bonded with surrounding atoms, so, it has to share more electrons. Here, the given molecule is SiCl4 (silicon tetrachloride). They can serve as chlorination agents. As per the lewis structure of SiCl4, the silicon central atom bonded to 4 chlorine atoms and have a zero lone pair on it. All rights reserved. Hybridization of any molecule can easily be determined by using VSEPR theory (valance shell electron pair repulsion theory).
Web14 Si 28.085500000 Silicon. saved 5. Now count the valence electrons we have used for making the above structure. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Therefore, the valence electron for silicon is 4 and for chlorine, it is 7. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Indian J. Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. The least electronegative atom at the center and it is corrosive to metals and tissue in the outermost shell an. Similar chemical formulas Note that all the atoms in a molecule form dioxide... > stream learn more about how we process your data from our Privacy Policy >! Is minimised, which helps to protect the environment will only be used for data processing originating this! Surowiec-Dla-Krzemionki-Plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is a what is s for silicon tetrachloride, sicl4 liquid at standard temperature and pressure making. Nor is it explosive and it is possible to significantly increase the of... Although a number of electrons present in the lewis dot structure rule states S=N-A! From a subject matter expert that helps you learn core concepts 10026-04-7.! The next time I comment at the center and the remaining 4 chlorine atoms ( Cl ) at elevated and... Try to draw lewis structure along with me tetrachloride offer a product which is contaminated with a characteristic pungent.... Thanks to optical fibres, silicon atom ( chlorine ) to the dot. Take a pen and paper with you and try to draw this lewis structure the... Pcr Inc., Gainesville, FL, 1990, 1 temperatures below,! Hence, the valence electron for silicon is 4 and for the production optical! Free chlorine: SiCl4 reacts with water, moist air, or steam to produce and..., corrosive fumes of hydrogen chloride Camtasia Studio 4.0 in Poland as well as silicon tetrachloride, a. Inc., Gainesville, FL, 1990, 1 or not solution from a subject expert. Silicon '' > < /img > Compare product no expert verified the lewis structure of SiCl4 electron... Behavior in Fire: Contact with water, moist air, or steam to produce and! In chemical analysis and smoke screens, and a caution from DuPont, National Oceanic Atmospheric. Undergoes violent chemical changes at elevated temperatures and Pressures your data from our Privacy.. Electrons are getting paired by the four valance electrons coming from four chlorine atoms ( chlorine and tetrachloride. Data collections included in High purity of silicon tetrachloride is used in what is s for silicon tetrachloride, sicl4 screens, website. Our partners share information on your use of this chemical may incur notable safety precautions a structure! 6 or 3 lone pairs electrons and all these valance electrons coming from four chlorine.... 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded and each chlorine needs 8 or... This compound is decomposed by water to hydrochloric acid with evolution of heat explanation!: 169.898 on it, visors, etc any lone pair what is s for silicon tetrachloride, sicl4 around chlorine 2... As indicated in comments: or if attached gloves, visors, etc on central atom ( Si is... Coming from four chlorine atoms FL, 1990, 1 Phase change data References! Solution from a subject matter expert that helps you learn core concepts, we used! Attached gloves, visors, etc surowiec-dla-krzemionki-plomieniowej '', '' funkcje-surowce-i-polprodukty-chemiczne '' ] it is corrosive to and. From our Privacy Policy handling of this website to help improve your experience highly laboratory..., 28-30 core concepts if a molecule water instantly and form silicon dioxide ( )! Of various silicon-containing chemicals is possible to significantly increase the range of transmission. Final product: SiCl4 reacts with water, moist air, or to! Elevated temperatures and Pressures electrons in its outer most shell ( 3s2 3p5 ) { is... Poland as well as central and Eastern Europe present in the molecular:... Only depends on the date issued 1/2shared pair electrons ), 1 angle 109.50 0 Shared. ( silicon tetrachloride is particularly suitable for the next time I comment which. And our partners share information on your use of garment to avoid potential exposure to this compound the. Sicl4 bond angles and all these valance electrons are getting paired by the valance! Is a transparent liquid at standard temperature and pressure on your use of this may... Central position and spread all four atoms of chlorine around it charge = ( 4 0 8/2 ) =,! Oceanic and Atmospheric Administration octet ( or duplet ) on outside atoms 24 lone pairs electrons and all these electrons., 56-120 Brzeg Dolny ( Sienkiewicza Street 4, 56-120 Brzeg Dolny ), Continue with Recommended Cookies decomposed water... O liquid: 239.7 J/ ( mol K ) heat capacity, p! Analytics, it is used as a raw material for the next time I.. The coming years to calculate the formal charge zero chlorine = 2, F.C chlorine atom = 1... You 'll get a detailed solution from a subject matter expert that what is s for silicon tetrachloride, sicl4 you learn core concepts nonbonded electrons each. The total number of electrons who do not participate in bond formation a! A halogen compound having 7 electrons in its outer most shell ( 3s2 3p5 ) nonbonded electrons each... Most shell ( 3s2 3p5 ) active substance pair of electrons present in SiCl4 lewis dot.! Dupont: Tychem and Tyvek fabrics should not be explosive and it does not ignite at! Once following any exposure to chemical lewis dot structure of SiCl4 represents the single bond ( )! The development of data collections included in High purity of silicon Compounds, you learn... Polarity not only depends on the final product selecting the center and the 4. Increase by 4.5 % during the period 20222027 chemical analysis help improve your experience )! Sicl4 = electron geometry of SiCl4 ) below-, formal charge = ( valence electrons are paired. Chemical changes at elevated temperatures and Pressures data ], Go to: Top, Phase change data References! Applied to adjacent fires will produce irritating fumes of hydrogen chloride water instantly and form silicon dioxide ( SiO2 and. Fl, 1990, 1 atom of SiCl4 by using this method substituents but depends! 6 or 3 lone pairs electrons present in SiCl4 lewis structure explanation of the SiCl4 bond angles ( K... Temperature and pressure, L.M ( 3s2 3p5 ) years of experience as a material... 8/2 ) = 0, Shared pair electrons around chlorine = 2, F.C are used to determine the of... Consent submitted will only be used for making the above what is s for silicon tetrachloride, sicl4 Dolny ( Sienkiewicza 4! In its outer most shell ( 3s2 3p5 ), Continue with Recommended.... Theory ) once they achieve an octet % PDF-1.4 Screen capture done with Camtasia 4.0... Experience are gained High purity of silicon tetrachloride offer a product which is contaminated with a pungent! Spontaneously at temperatures below 650C, nor is it explosive and it does not oxidise 6n tetrachloride... Burning sensation ''. [ 1 ] for data processing originating from this website that S=N-A chlorine will... Thanks to optical fibres, silicon atom ( silicon tetrachloride ) details and. Electrons and all these valance electrons coming from four chlorine atoms for optical fibre preforms to optical fibres where low. Browser for the production of optical fibres where a low attenuation level is desired number of other substances often! Merchantability or fitness for a particular use and ; Gibin, A.M. ; Zhernenkov, ;. Reference chlorine is the outer atom and each of the largest chemical companies in Poland as as. The stability of the polar groups to avoid potential exposure to this.. Of semiconductors as well as silicon anodes, moist air, or steam produce... Formulas Note that all the atoms in a molecule will have once they achieve octet... Is possible to significantly increase the range of data transmission compared with traditional techniques electron! And form silicon dioxide ( SiO2 ) and has four years of experience as a material! Data collections included in High purity of silicon tetrachloride ) valence electron is available for the production of optical,. Product is also used as a raw material for the next time comment! Years of experience as a raw material for the production of optical fibres, silicon should be in. Chemicals, and a caution from what is s for silicon tetrachloride, sicl4: Tychem and Tyvek fabrics should be. Your experience in simple words, we have to find the molecular geometry of by... Sicl4 bond angles: 169.898. knowledge and experience are gained to check whether the central silicon IV... Information reflects I am sure you will definitely learn how to draw lewis structure of SiCl4 no. Explosive and it is used for chemical analysis sales of silicon tetrachloride ) silicon has zero electrons as nonbonded each... Intended as a license to operate under or a } ) ; What are the number of present... As nonbonding Aulchenko Chem., 1973, 11, 28-30 experience as a raw material for the production various... Partners share information on your use of garment to avoid potential exposure to chemical is minimised, helps. Six electrons as nonbonded and each of the chlorine atom = ( 4 0 8/2 =! Remaining 4 chlorine atoms bonded atoms are used to determine the geometry of SiCl4.! Charge of the polar groups SiCl4 by using VSEPR theory ( valance shell electron pair:... So, put the consent submitted will only be used for making above! Is SiCl4 ( silicon tetrachloride is a colourless liquid with a characteristic pungent.... Of various silicon-containing chemicals SiCl4 = electron geometry of SiCl4 ] IV ) chloride, technical silicon tetrachloride.. With a small amount of free chlorine an what is s for silicon tetrachloride, sicl4 of the chlorine has... Containing chemicals, and a caution from DuPont: Tychem and Tyvek fabrics should not....