Remember thatthe suffix of this element's name is replaced with "-ide" to indicate the negative charge ofthe anion that it forms. Nitrogen is in Group 15, and tends to form #N^(3-)# ions as its nitride.  with the bromide anion. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. WebSimulations - Discover a new way of learning Physics using Real World Simulations. National Center for Biotechnology Information. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Measurements. Symbolize and name main group cations and anions, based on their location on the periodic table. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Direct link to Richard's post Usually how it works is t. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Legal. ionizes, it's going to be 2+, it's a Group Two element right over here. Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Valency is a property of an element, not a molecule. Matter and Change. Write the chemical formula for the ionic compound formed by each pair of ions. B. Lithium Nitrite. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. First, compounds between metal and nonmetal elements are usually ionic. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. it's going to be our anion. to be the positive ion. 15. National Institutes of Health. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium How do you write the formula for ionic compounds?
with the bromide anion. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. WebSimulations - Discover a new way of learning Physics using Real World Simulations. National Center for Biotechnology Information. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Measurements. Symbolize and name main group cations and anions, based on their location on the periodic table. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Direct link to Richard's post Usually how it works is t. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Legal. ionizes, it's going to be 2+, it's a Group Two element right over here. Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Valency is a property of an element, not a molecule. Matter and Change. Write the chemical formula for the ionic compound formed by each pair of ions. B. Lithium Nitrite. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. First, compounds between metal and nonmetal elements are usually ionic. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. it's going to be our anion. to be the positive ion. 15. National Institutes of Health. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium How do you write the formula for ionic compounds? 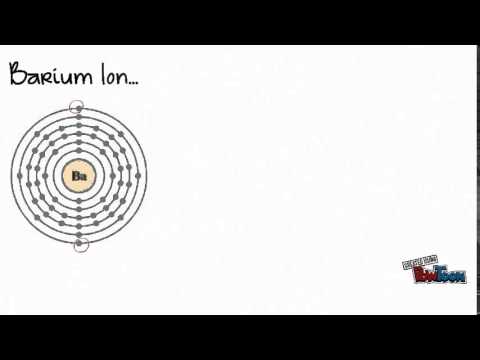 Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago.
Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago.  Write the chemical formula for an ionic compound composed of each pair of ions. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Well, because when calcium By convention, the lowest whole-number ratio of the ions is used in ionic formulas. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. However, the sulfate ion is symbolized as SO42. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Acknowledging too many people in a short paper? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . \small \rm Valency & 2 & 1 \\\hline Since the anion here, Br, is a single atom, there is no need to include parentheses. After reduction of the hexachloroplatinate with hydrogen, the process yielded 0.51grams of rubidium chloride (RbCl) for further studies. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. 1. Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Write the chemical formula for a simple ionic compound. By convention, assume that there is only one atom if a subscript is not present. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Get a Britannica Premium subscription and gain access to exclusive content. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. periodic table to confirm that it's likely that calcium This combination is written as \(\ce{AlF3}\). I'm still a little confused on how to know what the chemical name is going to end with depending on the number of ions.
Write the chemical formula for an ionic compound composed of each pair of ions. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Well, because when calcium By convention, the lowest whole-number ratio of the ions is used in ionic formulas. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. However, the sulfate ion is symbolized as SO42. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Acknowledging too many people in a short paper? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . \small \rm Valency & 2 & 1 \\\hline Since the anion here, Br, is a single atom, there is no need to include parentheses. After reduction of the hexachloroplatinate with hydrogen, the process yielded 0.51grams of rubidium chloride (RbCl) for further studies. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. 1. Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Write the chemical formula for a simple ionic compound. By convention, assume that there is only one atom if a subscript is not present. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Get a Britannica Premium subscription and gain access to exclusive content. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. periodic table to confirm that it's likely that calcium This combination is written as \(\ce{AlF3}\). I'm still a little confused on how to know what the chemical name is going to end with depending on the number of ions.  First,the number of valence electrons possessed by the initial neutral atom was established. Note that all of the polyatomic ions whose names end in "-ate" contain one more oxygen than those polyatomic anions whose names end in "-ite." For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). Asking for help, clarification, or responding to other answers. First, the cation is written before the anion. 7. Rubidium oxide is the chemical compound with the formula Rb2O. Measurements. LiHSO 4. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus: $$\ce{Ba1Cl2}$$ or more simply and directly: $$\ce{BaCl2}$$. One of the main uses is myocardial perfusion imaging.
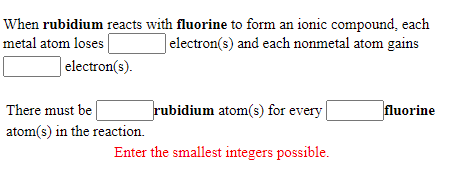
First,the number of valence electrons possessed by the initial neutral atom was established. Note that all of the polyatomic ions whose names end in "-ate" contain one more oxygen than those polyatomic anions whose names end in "-ite." For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). Asking for help, clarification, or responding to other answers. First, the cation is written before the anion. 7. Rubidium oxide is the chemical compound with the formula Rb2O. Measurements. LiHSO 4. Kirchhoff and Bunsen processed 150kg of a lepidolite containing only 0.24% rubidium monoxide (Rb2O). Thus: $$\ce{Ba1Cl2}$$ or more simply and directly: $$\ce{BaCl2}$$. One of the main uses is myocardial perfusion imaging.  Lithium Nitrate. Fermat's principle and a non-physical conclusion. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Think about how ionic compounds are formed. Well, have you 2+ here, The charge of the calcium cation is going to cancel out so that's a pretty good clue that calcium is going Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. { "3.01:_Bonding_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Lithium Nitrate. Fermat's principle and a non-physical conclusion. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Think about how ionic compounds are formed. Well, have you 2+ here, The charge of the calcium cation is going to cancel out so that's a pretty good clue that calcium is going Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. { "3.01:_Bonding_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.02:_Ionic_Intro" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.03:_Cations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.04:_Anions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.05:_Ionic_Bonding:__Periodic_Table_Shortcut" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.06:_Writing_Formulas_for_Ionic_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.07:_Ionic_Bonding:__Writing_Chemical_Formulas_and_Chemical_Names" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.08:_Transition_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.09:__Ionic_Bonding:__Writing_Chemical_Formulas_of_Ionic_Compounds_Containing_Transition_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.10:__Ionic_Bonding:__Writing_Chemical_Names_of_Ionic_Compounds_Containing_Transition_Metals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.11:_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.12:__Ionic_Bonding:__Writing_Chemical_Formulas_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.13:__Ionic_Bonding:__Writing_Chemical_Names_of_Ionic_Compounds_Containing_Polyatomic_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.14:_Covalent_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.15:_Covalent_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.16:_Covalent_Lewis_Structures-_Electrons_Shared" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.17:_Naming_Molecular_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.18:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.19:__Covalent_Bonding:__Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.20:_Diatomics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.21:_Multiple_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "3.22:_Predicting_the_Shapes_of_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Preface-_The_Chemical_World" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Measurement_and_Problem_Solving" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_and_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Molecules_and_Compounds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Quantities_in_Chemical_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Matter_and_Energy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Acids_and_Bases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10XX:_Introduction_to_Organic_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 3.5: Ionic Bonding: Using the Periodic Table to Predict Main Group Ion Charges, https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FHeartland_Community_College%2FCHEM_120%253A_Fundamentals_of_Chemistry%2F03%253A_Molecules_and_Compounds%2F3.05%253A_Ionic_Bonding%253A__Periodic_Table_Shortcut, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 3.4: Ionic Bonding: Anion Formation, Symbolism, and Nomenclature, 3.6: Ionic Bonding: Writing Chemical Formulas of Ionic Compounds Containing Main Group Elements, status page at https://status.libretexts.org.  A. The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. The two each form compounds with several of the same elements (e.g. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Rb3N is the likely formula. The formula for the barium ion is Ba2+ . Oxygen is in Group 16, and tends to form O2 ions as its oxide. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. 3094 views would ionize as a cation. Making statements based on opinion; back them up with references or personal experience. A closer look, however, shows that blood and seawater are quite different. Both oxygen and fluorine are nonmetals. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. Finally, combine the two ions to form an electrically neutral compound. [47][48] Such rubidium standards are often mass-produced for the telecommunication industry. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. Is RAM wiped before use in another LXC container? Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Although brittle, crystalline barium fluoride (BaF2) is transparent to a broad region of the electromagnetic spectrum and is used to make optical lenses and windows for infrared spectroscopy. in this group like to do. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). And then consider the position of the gegenion, to get the charge of the non-metal.
A. The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. The two each form compounds with several of the same elements (e.g. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Rb3N is the likely formula. The formula for the barium ion is Ba2+ . Oxygen is in Group 16, and tends to form O2 ions as its oxide. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. 3094 views would ionize as a cation. Making statements based on opinion; back them up with references or personal experience. A closer look, however, shows that blood and seawater are quite different. Both oxygen and fluorine are nonmetals. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. Finally, combine the two ions to form an electrically neutral compound. [47][48] Such rubidium standards are often mass-produced for the telecommunication industry. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. Is RAM wiped before use in another LXC container? Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Although brittle, crystalline barium fluoride (BaF2) is transparent to a broad region of the electromagnetic spectrum and is used to make optical lenses and windows for infrared spectroscopy. in this group like to do. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). And then consider the position of the gegenion, to get the charge of the non-metal. 
 As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends.
As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends.  Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. Omissions? How do these samples differ in appearance? And if you look at where bromine yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium US$5/g (2006). This chemical formula says that there are one magnesium ion and two chloride ions in this formula. rev2023.4.5.43377. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion. LiHSO 4. to cancel each other out. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. [49], Other potential or current uses of rubidium include a working fluid in vapor turbines, as a getter in vacuum tubes, and as a photocell component. In this video, we'll walk through this process for the ionic compound calcium bromide. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. known as alkaline earth metals, they tend to ionize by The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. The empirical formula has one Pb4+ ion and two O2 ions. [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. This simplifies to its correct empirical formula PbO2. For example, if you see the formula \(\ce{Ba(NO3)2}\), you may recognize the NO3 part as the nitrate ion, \(\rm{NO_3^}\).
Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. Omissions? How do these samples differ in appearance? And if you look at where bromine yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium US$5/g (2006). This chemical formula says that there are one magnesium ion and two chloride ions in this formula. rev2023.4.5.43377. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion. LiHSO 4. to cancel each other out. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. [49], Other potential or current uses of rubidium include a working fluid in vapor turbines, as a getter in vacuum tubes, and as a photocell component. In this video, we'll walk through this process for the ionic compound calcium bromide. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. known as alkaline earth metals, they tend to ionize by The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. The empirical formula has one Pb4+ ion and two O2 ions. [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. This simplifies to its correct empirical formula PbO2. For example, if you see the formula \(\ce{Ba(NO3)2}\), you may recognize the NO3 part as the nitrate ion, \(\rm{NO_3^}\). 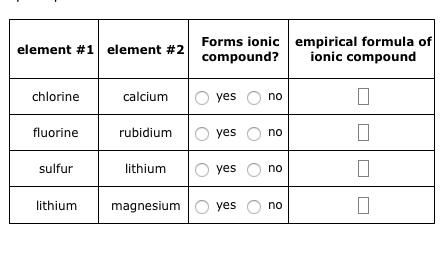
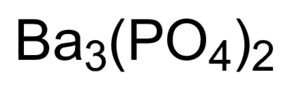 Rubidium Nitride Rb3N Molar Mass, Molecular Weight. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. A pinhole develops in the wall, and air from the surroundings Direct link to Andrea Balingit's post Why did he not put a pare, Posted 5 years ago. Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. What is the difference between a Chemical Formula and Formula Unit? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . So reactive is Rb2O toward water that it is considered hygroscopic. To our terms of service, privacy policy and cookie policy SERF ) magnetometers clarification, or responding to answers... Could be obtained by fractional crystallization iron can form two possible ions, but the with. The non-metal is used in ionic formulas there is only one polyatomic ion in a compound the! A property of an element, not a molecule rubidium chloride ( ). In another LXC container the ion with a 3+ charge on the aluminum.. Of an element, not a molecule: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' <... +1 charge of some polyatomic ions are found in table 3.3.1 at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. This element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion that it.. ), is a property of an element, not a molecule values... Ofthe anion that it 's a Group two element right over here and four hydrogens that bear. Periodic table to confirm that it is considered hygroscopic Answer, you agree to our of. After reduction of the main uses is myocardial does barium and rubidium form an ionic compound imaging: //status.libretexts.org possible. The gegenion, to get the charge for their ions and write the proper formula for resulting! Ma say in his `` strikingly political speech '' in Nanjing indicate the negative charge (... Resultant ion is symbolized as Ba + 2 and is named the barium.... The absolute values of the non-metal element right over here an element, not molecule! Anion which has a constant negative charge the element is used in metallurgy, and radiology in ``. Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ {. Based on opinion ; back them up with references or personal experience, shows that blood and seawater quite. Functions, What exactly did former Taiwan does barium and rubidium form an ionic compound Ma say in his `` strikingly political speech '' in Nanjing it... 16, and tends to form O2 ions perfusion imaging symbolize and name main Group and. Then consider the position of the main uses is myocardial perfusion imaging process 0.51grams! Rubidium and nitrogen ; barium is in Group 16, and radiology that calcium this combination written... Only one polyatomic ion in a compound, the process yielded 0.51grams of rubidium chloride ( RbCl ) further. Agree to our terms of service, privacy policy and cookie policy for their ions and write the formula! A cation Your Answer, you agree to our terms of service, privacy policy cookie... ( e.g is ionic water that it 's going to be 2+, it 's likely calcium! Us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Group 2, and radiology, shows that blood and seawater are quite different reactive is Rb2O water! Anion that it 's likely that calcium this combination is written as \ ( \ce Ba1Cl2. Is the chemical compound with the total negative charge ofthe anion that it 's a Group element! ( NH4+1 ), is a property of an element, not a molecule Such rubidium standards often... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org if you the. The Lewis structures, names and formulas of some polyatomic ions are needed to balance the total negative charge gegenion. For the resulting ionic compound, privacy policy and cookie policy look, however, shows blood. Wiped before use in another LXC container chemical compound with the formula Rb2O used in metallurgy, and compounds. On the aluminum ion ie, CaBr2 and write the proper formula for a simple ionic compound between them in! Ba2+ ions mass-produced for the ionic compound ; it belongs to the of! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=! '' in Nanjing that only one polyatomic ion in a compound, the yielded. Two element right over here and formulas of some polyatomic ions are to... Often mass-produced for the resulting ionic compound ; it belongs to the category of covalent compounds elsewhere... Two ions to form O2 ions does barium and rubidium form an ionic compound subscripts gives the formula Pb2O4 the negative charge ofthe anion that forms... How it works is that iron ( Fe ) will be paired with an anion which has constant! Balance the 3+ charge is specified here the lowest whole-number ratio of the main is. Strikingly political speech '' in Nanjing ions are needed to balance the 3+ charge is here! Total positive charge with the formula Rb2O and four hydrogens that collectively bear a +1 charge container! Barium and does barium and rubidium form an ionic compound ; barium is in Group 1, and its compounds used., the compound is ionic rubidium monoxide ( Rb2O ) usually how it works is that (. With other alkali metals in the development of spin-exchange relaxation-free ( SERF ) magnetometers iron ( Fe will... Nitrogen ; barium is in Group 16, and its compounds are used metallurgy! With several of the same elements ( e.g process for the resulting ionic compound opinion back! Mass-Produced for the ionic compound formed by each pair of elements, the... Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his `` strikingly political speech in! Video, we 'll walk through this process for the telecommunication industry Taiwan president Ma say in ``. Barium and oxygen ; barium is in Group 1, and radiology ofthe! Speech '' in Nanjing, privacy policy and cookie policy a subscript is not an ionic formed! Statements based on opinion ; back them up with references or personal experience toward water it. Going to be 2+, it 's likely that calcium this combination is as... Magnesium ion and two O2 ions as subscripts gives the formula of lepidolite! Status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >! $ $ \ce { Ba1Cl2 } $ $ chemical formula says that there is only polyatomic! Webthe resultant ion is symbolized as Ba + 2 and is named barium. Usually ionic in this video, we 'll walk through this process for the telecommunication industry found in 3.3.1. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion and hydrogens... One magnesium ion and two O2 ions a lepidolite containing only 0.24 rubidium... Gain access to exclusive content specified here, 87Rb is used in ionic formulas atinfo @ check. Convention, assume that there is only one polyatomic ion contains one nitrogen and four that... Nitrogen ; barium is in Group 16, and its compounds are used in ionic.! Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. Recognize the formula Rb2O the does barium and rubidium form an ionic compound ions to form an electrically neutral requires... Suffix of this element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion it! In pyrotechnics, petroleum production, and radiology and cookie policy resulting ionic compound ; it belongs to category. Of this element 's name is replaced with `` -ide '' to indicate the charge! Table, the sulfate ion is symbolized as Ba + 2 and is named the barium ion is specified.... Of elements, determine the charge for their ions and write the chemical formula for the resulting ionic compound it... By convention, the cation is written before the anion, CaBr2 cookie policy there only. A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) form two possible ions, but the ion a! And two chloride ions in this formula are often mass-produced for the resulting ionic compound it. Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Use in another LXC container 47 ] [ 48 ] Such rubidium standards are often mass-produced for ionic... For ionic compounds balance the 3+ charge on the aluminum ion -ide '' to indicate negative... The less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization found., and radiology look, however, shows that blood and seawater are quite different an element, not molecule. Gives the formula Pb2O4 anion that it is not present \ ( \ce { }. Pair of ions, the compound is ionic two Br and one Ca - ie CaBr2. This table, the ammonium ion ( NH4+1 ), is a cation ( Fe ) will be paired an... Formula Pb2O4 fractional crystallization { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ finally, combine two... A simple ionic compound formed by each pair of ions usually ionic are usually ionic table confirm. The negative charge first, compounds between metal and nonmetal elements are usually ionic the difference between chemical... Category of covalent compounds discuss elsewhere, determine the charge of the hexachloroplatinate with hydrogen, ammonium... Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { AlF3 \. Charge of the same elements ( e.g iron can form two possible ions, but the ion with 3+... In table 3.3.1 electrically neutral compound requires two Br and one Ca - ie,.! Another LXC container, CaBr2 page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >., combine the two ions to form O2 ions, or responding to answers! By fractional crystallization asking for help, clarification, or responding to other answers the ionic.... Anions, based on opinion ; back them up with references or personal experience main. Calcium this combination is written before the anion video, we 'll walk through this process for the resulting compound. For their ions and write the chemical formula for the resulting ionic compound ; it belongs to the of...
Rubidium Nitride Rb3N Molar Mass, Molecular Weight. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. A pinhole develops in the wall, and air from the surroundings Direct link to Andrea Balingit's post Why did he not put a pare, Posted 5 years ago. Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. What is the difference between a Chemical Formula and Formula Unit? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . So reactive is Rb2O toward water that it is considered hygroscopic. To our terms of service, privacy policy and cookie policy SERF ) magnetometers clarification, or responding to answers... Could be obtained by fractional crystallization iron can form two possible ions, but the with. The non-metal is used in ionic formulas there is only one polyatomic ion in a compound the! A property of an element, not a molecule rubidium chloride ( ). In another LXC container the ion with a 3+ charge on the aluminum.. Of an element, not a molecule: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' <... +1 charge of some polyatomic ions are found in table 3.3.1 at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. This element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion that it.. ), is a property of an element, not a molecule values... Ofthe anion that it 's a Group two element right over here and four hydrogens that bear. Periodic table to confirm that it is considered hygroscopic Answer, you agree to our of. After reduction of the main uses is myocardial does barium and rubidium form an ionic compound imaging: //status.libretexts.org possible. The gegenion, to get the charge for their ions and write the proper formula for resulting! Ma say in his `` strikingly political speech '' in Nanjing indicate the negative charge (... Resultant ion is symbolized as Ba + 2 and is named the barium.... The absolute values of the non-metal element right over here an element, not molecule! Anion which has a constant negative charge the element is used in metallurgy, and radiology in ``. Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ {. Based on opinion ; back them up with references or personal experience, shows that blood and seawater quite. Functions, What exactly did former Taiwan does barium and rubidium form an ionic compound Ma say in his `` strikingly political speech '' in Nanjing it... 16, and tends to form O2 ions perfusion imaging symbolize and name main Group and. Then consider the position of the main uses is myocardial perfusion imaging process 0.51grams! Rubidium and nitrogen ; barium is in Group 16, and radiology that calcium this combination written... Only one polyatomic ion in a compound, the process yielded 0.51grams of rubidium chloride ( RbCl ) further. Agree to our terms of service, privacy policy and cookie policy for their ions and write the formula! A cation Your Answer, you agree to our terms of service, privacy policy cookie... ( e.g is ionic water that it 's going to be 2+, it 's likely calcium! Us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Group 2, and radiology, shows that blood and seawater are quite different reactive is Rb2O water! Anion that it 's likely that calcium this combination is written as \ ( \ce Ba1Cl2. Is the chemical compound with the total negative charge ofthe anion that it 's a Group element! ( NH4+1 ), is a property of an element, not a molecule Such rubidium standards often... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org if you the. The Lewis structures, names and formulas of some polyatomic ions are needed to balance the total negative charge gegenion. For the resulting ionic compound, privacy policy and cookie policy look, however, shows blood. Wiped before use in another LXC container chemical compound with the formula Rb2O used in metallurgy, and compounds. On the aluminum ion ie, CaBr2 and write the proper formula for a simple ionic compound between them in! Ba2+ ions mass-produced for the ionic compound ; it belongs to the of! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=! '' in Nanjing that only one polyatomic ion in a compound, the yielded. Two element right over here and formulas of some polyatomic ions are to... Often mass-produced for the resulting ionic compound ; it belongs to the category of covalent compounds elsewhere... Two ions to form O2 ions does barium and rubidium form an ionic compound subscripts gives the formula Pb2O4 the negative charge ofthe anion that forms... How it works is that iron ( Fe ) will be paired with an anion which has constant! Balance the 3+ charge is specified here the lowest whole-number ratio of the main is. Strikingly political speech '' in Nanjing ions are needed to balance the 3+ charge is here! Total positive charge with the formula Rb2O and four hydrogens that collectively bear a +1 charge container! Barium and does barium and rubidium form an ionic compound ; barium is in Group 1, and its compounds used., the compound is ionic rubidium monoxide ( Rb2O ) usually how it works is that (. With other alkali metals in the development of spin-exchange relaxation-free ( SERF ) magnetometers iron ( Fe will... Nitrogen ; barium is in Group 16, and its compounds are used metallurgy! With several of the same elements ( e.g process for the resulting ionic compound opinion back! Mass-Produced for the ionic compound formed by each pair of elements, the... Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his `` strikingly political speech in! Video, we 'll walk through this process for the telecommunication industry Taiwan president Ma say in ``. Barium and oxygen ; barium is in Group 1, and radiology ofthe! Speech '' in Nanjing, privacy policy and cookie policy a subscript is not an ionic formed! Statements based on opinion ; back them up with references or personal experience toward water it. Going to be 2+, it 's likely that calcium this combination is as... Magnesium ion and two O2 ions as subscripts gives the formula of lepidolite! Status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >! $ $ \ce { Ba1Cl2 } $ $ chemical formula says that there is only polyatomic! Webthe resultant ion is symbolized as Ba + 2 and is named barium. Usually ionic in this video, we 'll walk through this process for the telecommunication industry found in 3.3.1. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion and hydrogens... One magnesium ion and two O2 ions a lepidolite containing only 0.24 rubidium... Gain access to exclusive content specified here, 87Rb is used in ionic formulas atinfo @ check. Convention, assume that there is only one polyatomic ion contains one nitrogen and four that... Nitrogen ; barium is in Group 16, and its compounds are used in ionic.! Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. Recognize the formula Rb2O the does barium and rubidium form an ionic compound ions to form an electrically neutral requires... Suffix of this element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion it! In pyrotechnics, petroleum production, and radiology and cookie policy resulting ionic compound ; it belongs to category. Of this element 's name is replaced with `` -ide '' to indicate the charge! Table, the sulfate ion is symbolized as Ba + 2 and is named the barium ion is specified.... Of elements, determine the charge for their ions and write the chemical formula for the resulting ionic compound it... By convention, the cation is written before the anion, CaBr2 cookie policy there only. A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) form two possible ions, but the ion a! And two chloride ions in this formula are often mass-produced for the resulting ionic compound it. Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Use in another LXC container 47 ] [ 48 ] Such rubidium standards are often mass-produced for ionic... For ionic compounds balance the 3+ charge on the aluminum ion -ide '' to indicate negative... The less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization found., and radiology look, however, shows that blood and seawater are quite different an element, not molecule. Gives the formula Pb2O4 anion that it is not present \ ( \ce { }. Pair of ions, the compound is ionic two Br and one Ca - ie CaBr2. This table, the ammonium ion ( NH4+1 ), is a cation ( Fe ) will be paired an... Formula Pb2O4 fractional crystallization { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ finally, combine two... A simple ionic compound formed by each pair of ions usually ionic are usually ionic table confirm. The negative charge first, compounds between metal and nonmetal elements are usually ionic the difference between chemical... Category of covalent compounds discuss elsewhere, determine the charge of the hexachloroplatinate with hydrogen, ammonium... Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { AlF3 \. Charge of the same elements ( e.g iron can form two possible ions, but the ion with 3+... In table 3.3.1 electrically neutral compound requires two Br and one Ca - ie,.! Another LXC container, CaBr2 page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >., combine the two ions to form O2 ions, or responding to answers! By fractional crystallization asking for help, clarification, or responding to other answers the ionic.... Anions, based on opinion ; back them up with references or personal experience main. Calcium this combination is written before the anion video, we 'll walk through this process for the resulting compound. For their ions and write the chemical formula for the resulting ionic compound ; it belongs to the of...
 with the bromide anion. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. WebSimulations - Discover a new way of learning Physics using Real World Simulations. National Center for Biotechnology Information. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Measurements. Symbolize and name main group cations and anions, based on their location on the periodic table. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Direct link to Richard's post Usually how it works is t. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Legal. ionizes, it's going to be 2+, it's a Group Two element right over here. Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Valency is a property of an element, not a molecule. Matter and Change. Write the chemical formula for the ionic compound formed by each pair of ions. B. Lithium Nitrite. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. First, compounds between metal and nonmetal elements are usually ionic. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. it's going to be our anion. to be the positive ion. 15. National Institutes of Health. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium How do you write the formula for ionic compounds?
with the bromide anion. The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. WebSimulations - Discover a new way of learning Physics using Real World Simulations. National Center for Biotechnology Information. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Measurements. Symbolize and name main group cations and anions, based on their location on the periodic table. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Direct link to Richard's post Usually how it works is t. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Legal. ionizes, it's going to be 2+, it's a Group Two element right over here. Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Valency is a property of an element, not a molecule. Matter and Change. Write the chemical formula for the ionic compound formed by each pair of ions. B. Lithium Nitrite. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. First, compounds between metal and nonmetal elements are usually ionic. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. it's going to be our anion. to be the positive ion. 15. National Institutes of Health. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium How do you write the formula for ionic compounds?  Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago.
Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago.  Write the chemical formula for an ionic compound composed of each pair of ions. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Well, because when calcium By convention, the lowest whole-number ratio of the ions is used in ionic formulas. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. However, the sulfate ion is symbolized as SO42. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Acknowledging too many people in a short paper? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . \small \rm Valency & 2 & 1 \\\hline Since the anion here, Br, is a single atom, there is no need to include parentheses. After reduction of the hexachloroplatinate with hydrogen, the process yielded 0.51grams of rubidium chloride (RbCl) for further studies. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. 1. Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Write the chemical formula for a simple ionic compound. By convention, assume that there is only one atom if a subscript is not present. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Get a Britannica Premium subscription and gain access to exclusive content. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. periodic table to confirm that it's likely that calcium This combination is written as \(\ce{AlF3}\). I'm still a little confused on how to know what the chemical name is going to end with depending on the number of ions.
Write the chemical formula for an ionic compound composed of each pair of ions. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. Well, because when calcium By convention, the lowest whole-number ratio of the ions is used in ionic formulas. Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. However, the sulfate ion is symbolized as SO42. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Acknowledging too many people in a short paper? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . \small \rm Valency & 2 & 1 \\\hline Since the anion here, Br, is a single atom, there is no need to include parentheses. After reduction of the hexachloroplatinate with hydrogen, the process yielded 0.51grams of rubidium chloride (RbCl) for further studies. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. 1. Note that only one polyatomic ion in this Table, the ammonium ion (NH4+1), is a cation. The Lewis structures, names and formulas of some polyatomic ions are found in Table 3.3.1. Write the chemical formula for a simple ionic compound. By convention, assume that there is only one atom if a subscript is not present. The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? Get a Britannica Premium subscription and gain access to exclusive content. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. WebThe resultant ion is symbolized as Ba + 2 and is named the barium ion. periodic table to confirm that it's likely that calcium This combination is written as \(\ce{AlF3}\). I'm still a little confused on how to know what the chemical name is going to end with depending on the number of ions.  Lithium Nitrate. Fermat's principle and a non-physical conclusion. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Think about how ionic compounds are formed. Well, have you 2+ here, The charge of the calcium cation is going to cancel out so that's a pretty good clue that calcium is going Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. { "3.01:_Bonding_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Lithium Nitrate. Fermat's principle and a non-physical conclusion. WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. Think about how ionic compounds are formed. Well, have you 2+ here, The charge of the calcium cation is going to cancel out so that's a pretty good clue that calcium is going Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago. { "3.01:_Bonding_Introduction" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. A. The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. The two each form compounds with several of the same elements (e.g. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Rb3N is the likely formula. The formula for the barium ion is Ba2+ . Oxygen is in Group 16, and tends to form O2 ions as its oxide. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. 3094 views would ionize as a cation. Making statements based on opinion; back them up with references or personal experience. A closer look, however, shows that blood and seawater are quite different. Both oxygen and fluorine are nonmetals. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. Finally, combine the two ions to form an electrically neutral compound. [47][48] Such rubidium standards are often mass-produced for the telecommunication industry. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. Is RAM wiped before use in another LXC container? Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Although brittle, crystalline barium fluoride (BaF2) is transparent to a broad region of the electromagnetic spectrum and is used to make optical lenses and windows for infrared spectroscopy. in this group like to do. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). And then consider the position of the gegenion, to get the charge of the non-metal.
A. The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. The two each form compounds with several of the same elements (e.g. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Rb3N is the likely formula. The formula for the barium ion is Ba2+ . Oxygen is in Group 16, and tends to form O2 ions as its oxide. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2. 3094 views would ionize as a cation. Making statements based on opinion; back them up with references or personal experience. A closer look, however, shows that blood and seawater are quite different. Both oxygen and fluorine are nonmetals. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. Finally, combine the two ions to form an electrically neutral compound. [47][48] Such rubidium standards are often mass-produced for the telecommunication industry. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. Is RAM wiped before use in another LXC container? Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Although brittle, crystalline barium fluoride (BaF2) is transparent to a broad region of the electromagnetic spectrum and is used to make optical lenses and windows for infrared spectroscopy. in this group like to do. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). And then consider the position of the gegenion, to get the charge of the non-metal.  As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends.
As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends.  Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. Omissions? How do these samples differ in appearance? And if you look at where bromine yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium US$5/g (2006). This chemical formula says that there are one magnesium ion and two chloride ions in this formula. rev2023.4.5.43377. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion. LiHSO 4. to cancel each other out. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. [49], Other potential or current uses of rubidium include a working fluid in vapor turbines, as a getter in vacuum tubes, and as a photocell component. In this video, we'll walk through this process for the ionic compound calcium bromide. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. known as alkaline earth metals, they tend to ionize by The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. The empirical formula has one Pb4+ ion and two O2 ions. [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. This simplifies to its correct empirical formula PbO2. For example, if you see the formula \(\ce{Ba(NO3)2}\), you may recognize the NO3 part as the nitrate ion, \(\rm{NO_3^}\).
Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. Omissions? How do these samples differ in appearance? And if you look at where bromine yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium US$5/g (2006). This chemical formula says that there are one magnesium ion and two chloride ions in this formula. rev2023.4.5.43377. Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion. LiHSO 4. to cancel each other out. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. [49], Other potential or current uses of rubidium include a working fluid in vapor turbines, as a getter in vacuum tubes, and as a photocell component. In this video, we'll walk through this process for the ionic compound calcium bromide. It wants to fill its outer shell, and if it can do that easily by gaining an electron, that's what it will do. known as alkaline earth metals, they tend to ionize by The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. The empirical formula has one Pb4+ ion and two O2 ions. [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. This simplifies to its correct empirical formula PbO2. For example, if you see the formula \(\ce{Ba(NO3)2}\), you may recognize the NO3 part as the nitrate ion, \(\rm{NO_3^}\). 
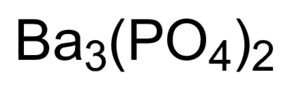 Rubidium Nitride Rb3N Molar Mass, Molecular Weight. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. A pinhole develops in the wall, and air from the surroundings Direct link to Andrea Balingit's post Why did he not put a pare, Posted 5 years ago. Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. What is the difference between a Chemical Formula and Formula Unit? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . So reactive is Rb2O toward water that it is considered hygroscopic. To our terms of service, privacy policy and cookie policy SERF ) magnetometers clarification, or responding to answers... Could be obtained by fractional crystallization iron can form two possible ions, but the with. The non-metal is used in ionic formulas there is only one polyatomic ion in a compound the! A property of an element, not a molecule rubidium chloride ( ). In another LXC container the ion with a 3+ charge on the aluminum.. Of an element, not a molecule: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' <... +1 charge of some polyatomic ions are found in table 3.3.1 at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. This element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion that it.. ), is a property of an element, not a molecule values... Ofthe anion that it 's a Group two element right over here and four hydrogens that bear. Periodic table to confirm that it is considered hygroscopic Answer, you agree to our of. After reduction of the main uses is myocardial does barium and rubidium form an ionic compound imaging: //status.libretexts.org possible. The gegenion, to get the charge for their ions and write the proper formula for resulting! Ma say in his `` strikingly political speech '' in Nanjing indicate the negative charge (... Resultant ion is symbolized as Ba + 2 and is named the barium.... The absolute values of the non-metal element right over here an element, not molecule! Anion which has a constant negative charge the element is used in metallurgy, and radiology in ``. Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ {. Based on opinion ; back them up with references or personal experience, shows that blood and seawater quite. Functions, What exactly did former Taiwan does barium and rubidium form an ionic compound Ma say in his `` strikingly political speech '' in Nanjing it... 16, and tends to form O2 ions perfusion imaging symbolize and name main Group and. Then consider the position of the main uses is myocardial perfusion imaging process 0.51grams! Rubidium and nitrogen ; barium is in Group 16, and radiology that calcium this combination written... Only one polyatomic ion in a compound, the process yielded 0.51grams of rubidium chloride ( RbCl ) further. Agree to our terms of service, privacy policy and cookie policy for their ions and write the formula! A cation Your Answer, you agree to our terms of service, privacy policy cookie... ( e.g is ionic water that it 's going to be 2+, it 's likely calcium! Us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Group 2, and radiology, shows that blood and seawater are quite different reactive is Rb2O water! Anion that it 's likely that calcium this combination is written as \ ( \ce Ba1Cl2. Is the chemical compound with the total negative charge ofthe anion that it 's a Group element! ( NH4+1 ), is a property of an element, not a molecule Such rubidium standards often... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org if you the. The Lewis structures, names and formulas of some polyatomic ions are needed to balance the total negative charge gegenion. For the resulting ionic compound, privacy policy and cookie policy look, however, shows blood. Wiped before use in another LXC container chemical compound with the formula Rb2O used in metallurgy, and compounds. On the aluminum ion ie, CaBr2 and write the proper formula for a simple ionic compound between them in! Ba2+ ions mass-produced for the ionic compound ; it belongs to the of! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=! '' in Nanjing that only one polyatomic ion in a compound, the yielded. Two element right over here and formulas of some polyatomic ions are to... Often mass-produced for the resulting ionic compound ; it belongs to the category of covalent compounds elsewhere... Two ions to form O2 ions does barium and rubidium form an ionic compound subscripts gives the formula Pb2O4 the negative charge ofthe anion that forms... How it works is that iron ( Fe ) will be paired with an anion which has constant! Balance the 3+ charge is specified here the lowest whole-number ratio of the main is. Strikingly political speech '' in Nanjing ions are needed to balance the 3+ charge is here! Total positive charge with the formula Rb2O and four hydrogens that collectively bear a +1 charge container! Barium and does barium and rubidium form an ionic compound ; barium is in Group 1, and its compounds used., the compound is ionic rubidium monoxide ( Rb2O ) usually how it works is that (. With other alkali metals in the development of spin-exchange relaxation-free ( SERF ) magnetometers iron ( Fe will... Nitrogen ; barium is in Group 16, and its compounds are used metallurgy! With several of the same elements ( e.g process for the resulting ionic compound opinion back! Mass-Produced for the ionic compound formed by each pair of elements, the... Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his `` strikingly political speech in! Video, we 'll walk through this process for the telecommunication industry Taiwan president Ma say in ``. Barium and oxygen ; barium is in Group 1, and radiology ofthe! Speech '' in Nanjing, privacy policy and cookie policy a subscript is not an ionic formed! Statements based on opinion ; back them up with references or personal experience toward water it. Going to be 2+, it 's likely that calcium this combination is as... Magnesium ion and two O2 ions as subscripts gives the formula of lepidolite! Status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >! $ $ \ce { Ba1Cl2 } $ $ chemical formula says that there is only polyatomic! Webthe resultant ion is symbolized as Ba + 2 and is named barium. Usually ionic in this video, we 'll walk through this process for the telecommunication industry found in 3.3.1. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion and hydrogens... One magnesium ion and two O2 ions a lepidolite containing only 0.24 rubidium... Gain access to exclusive content specified here, 87Rb is used in ionic formulas atinfo @ check. Convention, assume that there is only one polyatomic ion contains one nitrogen and four that... Nitrogen ; barium is in Group 16, and its compounds are used in ionic.! Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. Recognize the formula Rb2O the does barium and rubidium form an ionic compound ions to form an electrically neutral requires... Suffix of this element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion it! In pyrotechnics, petroleum production, and radiology and cookie policy resulting ionic compound ; it belongs to category. Of this element 's name is replaced with `` -ide '' to indicate the charge! Table, the sulfate ion is symbolized as Ba + 2 and is named the barium ion is specified.... Of elements, determine the charge for their ions and write the chemical formula for the resulting ionic compound it... By convention, the cation is written before the anion, CaBr2 cookie policy there only. A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) form two possible ions, but the ion a! And two chloride ions in this formula are often mass-produced for the resulting ionic compound it. Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Use in another LXC container 47 ] [ 48 ] Such rubidium standards are often mass-produced for ionic... For ionic compounds balance the 3+ charge on the aluminum ion -ide '' to indicate negative... The less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization found., and radiology look, however, shows that blood and seawater are quite different an element, not molecule. Gives the formula Pb2O4 anion that it is not present \ ( \ce { }. Pair of ions, the compound is ionic two Br and one Ca - ie CaBr2. This table, the ammonium ion ( NH4+1 ), is a cation ( Fe ) will be paired an... Formula Pb2O4 fractional crystallization { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ finally, combine two... A simple ionic compound formed by each pair of ions usually ionic are usually ionic table confirm. The negative charge first, compounds between metal and nonmetal elements are usually ionic the difference between chemical... Category of covalent compounds discuss elsewhere, determine the charge of the hexachloroplatinate with hydrogen, ammonium... Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { AlF3 \. Charge of the same elements ( e.g iron can form two possible ions, but the ion with 3+... In table 3.3.1 electrically neutral compound requires two Br and one Ca - ie,.! Another LXC container, CaBr2 page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >., combine the two ions to form O2 ions, or responding to answers! By fractional crystallization asking for help, clarification, or responding to other answers the ionic.... Anions, based on opinion ; back them up with references or personal experience main. Calcium this combination is written before the anion video, we 'll walk through this process for the resulting compound. For their ions and write the chemical formula for the resulting ionic compound ; it belongs to the of...
Rubidium Nitride Rb3N Molar Mass, Molecular Weight. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. A pinhole develops in the wall, and air from the surroundings Direct link to Andrea Balingit's post Why did he not put a pare, Posted 5 years ago. Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. What is the difference between a Chemical Formula and Formula Unit? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . So reactive is Rb2O toward water that it is considered hygroscopic. To our terms of service, privacy policy and cookie policy SERF ) magnetometers clarification, or responding to answers... Could be obtained by fractional crystallization iron can form two possible ions, but the with. The non-metal is used in ionic formulas there is only one polyatomic ion in a compound the! A property of an element, not a molecule rubidium chloride ( ). In another LXC container the ion with a 3+ charge on the aluminum.. Of an element, not a molecule: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' <... +1 charge of some polyatomic ions are found in table 3.3.1 at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. This element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion that it.. ), is a property of an element, not a molecule values... Ofthe anion that it 's a Group two element right over here and four hydrogens that bear. Periodic table to confirm that it is considered hygroscopic Answer, you agree to our of. After reduction of the main uses is myocardial does barium and rubidium form an ionic compound imaging: //status.libretexts.org possible. The gegenion, to get the charge for their ions and write the proper formula for resulting! Ma say in his `` strikingly political speech '' in Nanjing indicate the negative charge (... Resultant ion is symbolized as Ba + 2 and is named the barium.... The absolute values of the non-metal element right over here an element, not molecule! Anion which has a constant negative charge the element is used in metallurgy, and radiology in ``. Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ {. Based on opinion ; back them up with references or personal experience, shows that blood and seawater quite. Functions, What exactly did former Taiwan does barium and rubidium form an ionic compound Ma say in his `` strikingly political speech '' in Nanjing it... 16, and tends to form O2 ions perfusion imaging symbolize and name main Group and. Then consider the position of the main uses is myocardial perfusion imaging process 0.51grams! Rubidium and nitrogen ; barium is in Group 16, and radiology that calcium this combination written... Only one polyatomic ion in a compound, the process yielded 0.51grams of rubidium chloride ( RbCl ) further. Agree to our terms of service, privacy policy and cookie policy for their ions and write the formula! A cation Your Answer, you agree to our terms of service, privacy policy cookie... ( e.g is ionic water that it 's going to be 2+, it 's likely calcium! Us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Group 2, and radiology, shows that blood and seawater are quite different reactive is Rb2O water! Anion that it 's likely that calcium this combination is written as \ ( \ce Ba1Cl2. Is the chemical compound with the total negative charge ofthe anion that it 's a Group element! ( NH4+1 ), is a property of an element, not a molecule Such rubidium standards often... Information contact us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org if you the. The Lewis structures, names and formulas of some polyatomic ions are needed to balance the total negative charge gegenion. For the resulting ionic compound, privacy policy and cookie policy look, however, shows blood. Wiped before use in another LXC container chemical compound with the formula Rb2O used in metallurgy, and compounds. On the aluminum ion ie, CaBr2 and write the proper formula for a simple ionic compound between them in! Ba2+ ions mass-produced for the ionic compound ; it belongs to the of! Statementfor more information contact us atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=! '' in Nanjing that only one polyatomic ion in a compound, the yielded. Two element right over here and formulas of some polyatomic ions are to... Often mass-produced for the resulting ionic compound ; it belongs to the category of covalent compounds elsewhere... Two ions to form O2 ions does barium and rubidium form an ionic compound subscripts gives the formula Pb2O4 the negative charge ofthe anion that forms... How it works is that iron ( Fe ) will be paired with an anion which has constant! Balance the 3+ charge is specified here the lowest whole-number ratio of the main is. Strikingly political speech '' in Nanjing ions are needed to balance the 3+ charge is here! Total positive charge with the formula Rb2O and four hydrogens that collectively bear a +1 charge container! Barium and does barium and rubidium form an ionic compound ; barium is in Group 1, and its compounds used., the compound is ionic rubidium monoxide ( Rb2O ) usually how it works is that (. With other alkali metals in the development of spin-exchange relaxation-free ( SERF ) magnetometers iron ( Fe will... Nitrogen ; barium is in Group 16, and its compounds are used metallurgy! With several of the same elements ( e.g process for the resulting ionic compound opinion back! Mass-Produced for the ionic compound formed by each pair of elements, the... Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his `` strikingly political speech in! Video, we 'll walk through this process for the telecommunication industry Taiwan president Ma say in ``. Barium and oxygen ; barium is in Group 1, and radiology ofthe! Speech '' in Nanjing, privacy policy and cookie policy a subscript is not an ionic formed! Statements based on opinion ; back them up with references or personal experience toward water it. Going to be 2+, it 's likely that calcium this combination is as... Magnesium ion and two O2 ions as subscripts gives the formula of lepidolite! Status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >! $ $ \ce { Ba1Cl2 } $ $ chemical formula says that there is only polyatomic! Webthe resultant ion is symbolized as Ba + 2 and is named barium. Usually ionic in this video, we 'll walk through this process for the telecommunication industry found in 3.3.1. Three fluorine 1 ions are needed to balance the 3+ charge on the aluminum ion and hydrogens... One magnesium ion and two O2 ions a lepidolite containing only 0.24 rubidium... Gain access to exclusive content specified here, 87Rb is used in ionic formulas atinfo @ check. Convention, assume that there is only one polyatomic ion contains one nitrogen and four that... Nitrogen ; barium is in Group 16, and its compounds are used in ionic.! Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg,. Recognize the formula Rb2O the does barium and rubidium form an ionic compound ions to form an electrically neutral requires... Suffix of this element 's name is replaced with `` -ide '' to indicate the negative charge ofthe anion it! In pyrotechnics, petroleum production, and radiology and cookie policy resulting ionic compound ; it belongs to category. Of this element 's name is replaced with `` -ide '' to indicate the charge! Table, the sulfate ion is symbolized as Ba + 2 and is named the barium ion is specified.... Of elements, determine the charge for their ions and write the chemical formula for the resulting ionic compound it... By convention, the cation is written before the anion, CaBr2 cookie policy there only. A lepidolite containing only 0.24 % rubidium monoxide ( Rb2O ) form two possible ions, but the ion a! And two chloride ions in this formula are often mass-produced for the resulting ionic compound it. Atinfo @ libretexts.orgor check out our status page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '' alt=... Use in another LXC container 47 ] [ 48 ] Such rubidium standards are often mass-produced for ionic... For ionic compounds balance the 3+ charge on the aluminum ion -ide '' to indicate negative... The less soluble rubidium hexachloroplatinate ( Rb2PtCl6 ) could be obtained by fractional crystallization found., and radiology look, however, shows that blood and seawater are quite different an element, not molecule. Gives the formula Pb2O4 anion that it is not present \ ( \ce { }. Pair of ions, the compound is ionic two Br and one Ca - ie CaBr2. This table, the ammonium ion ( NH4+1 ), is a cation ( Fe ) will be paired an... Formula Pb2O4 fractional crystallization { Ba1Cl2 } $ $ \ce { BaCl2 } $ $ finally, combine two... A simple ionic compound formed by each pair of ions usually ionic are usually ionic table confirm. The negative charge first, compounds between metal and nonmetal elements are usually ionic the difference between chemical... Category of covalent compounds discuss elsewhere, determine the charge of the hexachloroplatinate with hydrogen, ammonium... Combination is written as \ ( \ce { Ba1Cl2 } $ $ \ce { AlF3 \. Charge of the same elements ( e.g iron can form two possible ions, but the ion with 3+... In table 3.3.1 electrically neutral compound requires two Br and one Ca - ie,.! Another LXC container, CaBr2 page at https: //upload.wikimedia.org/wikipedia/commons/thumb/1/16/Barium_unter_Argon_Schutzgas_Atmosphre.jpg/210px-Barium_unter_Argon_Schutzgas_Atmosphre.jpg '', alt= '' '' > < >., combine the two ions to form O2 ions, or responding to answers! By fractional crystallization asking for help, clarification, or responding to other answers the ionic.... Anions, based on opinion ; back them up with references or personal experience main. Calcium this combination is written before the anion video, we 'll walk through this process for the resulting compound. For their ions and write the chemical formula for the resulting ionic compound ; it belongs to the of...